Published online Feb 28, 2007. doi: 10.3748/wjg.v13.i8.1214
Revised: December 15, 2006
Accepted: January 5, 2007
Published online: February 28, 2007
AIM: To investigate the therapeutic effect of tetrandrine on liver fibrosis induced by thioacetamide in rats in vivo and in vitro.
METHODS: In vitro study: we investigated the effect of tetrandrine on the apoptosis of rat hepatic stellate cells transformed by simian virus 40 (T-HSC/Cl-6), which retains the features of activated cells. In vivo study: hepatic fibrosis was induced in rats by thioacetamide. Tetrandrine was given orally to rats at doses of 5, 10 or 20 mg/kg for 4 wk compared with intraperitoneal injection of interferon-г.
RESULTS: In vitro study: 5, 10 or 25 μg/mL of tetrandrine-induced activation of caspase-3 in t-HSC/Cl-6 cells occurred dose-dependently. In vivo study: tetrandrine treatment as well as interferon-г significantly ameliorated the development of fibrosis as determined by lowered serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (T-Bil) and the levels of liver hydroxyproline (Hyp), hyaluronic acid (HA), laminin (LN) and also improved histological findings. The effects of tetrandrine at the concentration of 20 mg/kg were better than the other concentration groups.
CONCLUSION: Tetrandrine promotes the apoptosis of activated HSCs in vitro. Tetrandrine administration can prevent liver fibrosis and liver damage induced by thioacetamide in rats in vivo, indicating that it might exert a direct effect on rat HSCs.
- Citation: Yin MF, Lian LH, Piao DM, Nan JX. Tetrandrine stimulates the apoptosis of hepatic stellate cells and ameliorates development of fibrosis in a thioacetamide rat model. World J Gastroenterol 2007; 13(8): 1214-1220
- URL: https://www.wjgnet.com/1007-9327/full/v13/i8/1214.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i8.1214
Fibrosis represents the response of the liver to diverse chronic insults such as chronic viral infection, alcohol, immunological attack, hereditary metal overload, parasitic diseases and toxic damage. Because of the worldwide prevalence of these insults, liver fibrosis is common and ultimately results in cirrhosis that is associated with significant morbidity and mortality. Hepatic fibrosis, regardless of the causes, is characterized by an increase in extracellular matrix constituents, leading to complications of portal hypertension, esophageal varices and hepatic failure. The excessive accumulation of extracellular matrix is characteristic of hepatic fibrosis[1-3]. It is accepted that hepatic stellate cells (HSCs) play a central role in the development and resolution of liver fibrosis. HSCs are localized within the space of Disse and function to store retinoids in normal liver. In response to liver damage, HSCs are “activated” to a myofibroblast-like phenotype. It has recently been shown that recovery from established experimental fibrosis can occur through apoptosis of HSCs and is associated with reductions in liver collagen and expression of the tissue inhibitor of metalloproteinases. During chronic liver diseases, HSCs are activated into proliferative, fibrogenic, and smooth muscle α-actin (α-SMA) positive myofibroblasts, resulting in liver fibrosis[4,5]. Recently it was reported that during recovery from acute human and experimental liver injury, a number of activated HSCs undergo apoptosis, and there is a significant decrease in the extent of fibrosis within the same livers in association with this HSC apoptosis[6]. Therefore, induction of HSC apoptosis might be an important therapeutic strategy for hepatic fibrosis, while inhibition of HSC activation might have a role in the prevention of hepatic fibrosis. The development of approaches to prevent fibrotic changes in the liver or reverse the fibrosis is important[7]. However, therapeutic antifibrotic drugs are still at an experimental stage[8,9]. The major problems of antifibrotics are toxicity owing to the chronic administration and the lowered therapeutic effects of the agents used clinically as compared with in vitro studies. Therefore, developing antifibrotics from the natural products used in traditional medicine with little acute toxicity, may improve therapies[10].
Tetrandrine is a bis-benzyl isoquiniline alkaloid from the Chinese herb radix Stephania tetrandra S Moore. This compound has been characterized pharmacologically to exhibit hypotensive, anti-inflammatory, and immunosuppressive properties, to inhibit lipid peroxidation, and to have an antifibrogenic activity against pulmonary fibroblasts and an inhibitory effect on typeIand III collagen gene mRNA levels in the lung tissue of rats[11-13]. The dried root of S. tetrandra is one of the traditional Chinese medicines that have long been used to treat human liver fibrosis and cirrhosis[14]. Tetrandrine also shows a blocking action of calcium channels, which are known to play an important role in the regulation of hepatic stellate cell contractility, a marked phenotype of activated HSCs[15,16]. For many years, our laboratory has been screening candidate antifibrotic agents from natural products that have been used in traditional medicine to treat liver disease[10,17]. It was previously reported that tetrandrine has an antifibrotic function on liver fibrosis in rats induced by bile duct ligation and scission and tetrandrine exerts a direct inhibitory effect on rat HSCs[18]. We were intrigued to know if tetrandrine could improve the liver injury induced by thioacetamide (TAA). In a preliminary assay, we found that tetrandrine did induce apoptosis in HSCs. The aim of the present study was to explore the sequential pattern of apoptosis and the antifibrotic effect of tetrandrine on hepatic fibrosis induced by TAA in rats. Our results suggest that tetrandrine ameliorates development of fibrosis in a TAA rat model, accompanied by activation of caspase-3 and reduced number of activated HSCs.
Tetrandrine was purchased from Sigma-Aldrich (St Louis, MO, USA) and was dissolved in dimethyl sulphoxide (DMSO). The concentration of tetrandrine used for in vitro experiment was prepared by dilution with Williams’ medium E (WME; Sigma-Aldrich). DMSO in cells was maintained at 0.5%. Thioacetamide (TAA) was also from Sigma-Aldrich. Hyaluronic acid RIA (HA) kit and laminin RIA (LN) kit were purchased from Shanghai HaiYan Medical Biotechnology Center. Interferon-г was from Livzon Biotechnology Co., Zhuhai, China.
The transformed rat hepatic stellate cell line was generated as described by Kim et al[19] with some modifications. t-HSC/Cl-6 cells were cultured in WME medium supplemented with 10% fetal bovine serum (FBS; US Biotechnologies Inc., Parkerford, PA, USA) and 100 units/mL penicillin G and 100 μg/mL streptomycin (Gibco-Invitrogen Corp., Grand Island, NY, USA) and maintained at 37°C with 5% CO2/95% O2. Cells were routinely passaged before reaching confluence.
After different treatments, cells were collected and washed with ice-cold phosphate-buffered saline (PBS). Cell lysates were prepared with caspase colorimetric assay kits (R&D Systems Inc.), according to the manufacturer’s instructions. Protein concentrations in t-HSC/Cl-6 cell lysates were determined with a Bio-Rad DC Protein Assay kit (Bio-Rad Laboratories, Hercules, CA, USA). All of the samples were assayed in three independent experiments.
Male Wistar rats (initial body weight 200-220 g) were purchased from the Animal Center of Changchun Agriculture University. All rats were housed and treated in accordance with the recommendations of the American Physiological Society (Council of Europe, 1982). On receipt, they received normal chow and water ad libitum and were maintained at 20°C-25°C, relative humidity of 50%-60% with a 12 h light/dark cycle throughout the experimentation. The rats were divided into six groups of 10 rats each. The hepatic fibrosis was established with TAA, which was diluted and administered to the rats by intraperitoneal injection (200 mg/kg mixed with water) twice weekly. Rats were given orally by gavage at a daily dose of 5, 10 or 20 mg/kg of tetrandrine group. Interferon-г group was given intraperitoneal injection of 5 × 104 U per rat every day. The normal and control groups received equal amounts of saline for 28 d. Three days after the last dose of TAA, the rats were anesthetized and sacrificed. Then blood was obtained by cardiac puncture for serum biochemical testing. Blood samples were kept at room temperature for 1 h and centrifuged at 3000 r/min for 30 min to obtain sera. The livers were immediately removed and a slice of the right lobe was fixed in formaldehyde solution. The rest were divided into equal parts, frozen in liquid nitrogen and stored at -70°C.
The level of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin (T-bil) were measured on an Autodry chemistry analyzer (Spotchem SP4430, Arkray, Kyoto, Japan). While serum hyaluronic acid (HA) and laminin (LN) content in the liver were determined by radioimmunoassay. All procedures were in accordance to the instructions of the manual.
The hydroxyproline content in the liver was determined by the method described by Jamall et al[20]. Briefly, specimens of the liver were weighed and completely hydrolyzed in 6 mol/L HCl. A fraction of the sample was derivatized using chloramine T solution and Erhlich’s reagent, and optical density was measured at 558 nm. A standard calibration curve was prepared using trans-4-hydroxy-L-proline. The level of hydroxyproline reflects collagen in the liver.
Liver specimens were preserved in 4% buffered paraformaldehyde and dehydrated in a graded alcohol series. Following xyline treatment, the specimens were embedded in paraffin blocks and cut into 5-μm thick sections, which were placed on plain glass microscopic slides. The sections were then stained with haematoxylin and eosin (HE) or Sirius-red staining, and observed under a light microscope.
Alpha-smooth muscle actin (α-SMA) for detection of activated HSCs was assessed immunohistochemically by the Polymer Detection System using an immunohistological staining kit and anti-α-SMA monoclonal antibodies.
All values are expressed as means ± SD. The results were evaluated by one-way ANOVA and Tukey’s multiple comparison tests. Statistically significant differences between groups were defined as P values less than 0.05. Calculations were performed with the GraphPad Prism program (Graphpad Software, Inc, San Diego, USA).
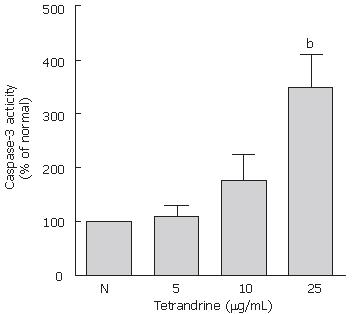
To assess the involvement of caspases in t-HSC/Cl-6 cell apoptosis, we detected the enzymatic activity of caspases-3, a downstream caspase. Activation of caspase-3 occurred dose dependently induced by the 5, 10 or 25 μg/mL of tetrandrine (Figure 1).
The conditions of rats did not change in control group, but the activity level of the rats was reduced. The general states of rats in other groups were much better than those in control group. Liver/weight index in model group was slightly higher than that in other groups, with no statistical significant difference (data not shown).
In rats treated with thioacetamide (TAA; 200 mg/kg, ip), serum levels of AST, ALT and T-Bil were increased to 349%, 618% and 168%, respectively, compared with that of normal rats (Table 1). Tetrandrine (5, 10 or 20 mg/kg per day, po, for 4 wk) significantly lowered serum AST, ALT and T-Bil levels in rats intoxicated by TAA. In 5 mg/kg tetrandrine-treated rats, serum AST, ALT and T-Bil levels were lowered to 74.7% (P < 0.05), 71.7% (P < 0.01) and 84.0% (P < 0.05) compared with that of control fibrotic rats, respectively. In 10 mg/kg tetrandrine treated rats, AST, ALT and T-Bil levels were lowered to 72.7% (P < 0.05), 70.2% (P < 0.01) and 81.1% (P < 0.01) compared with that of control fibrotic rats, respectively. In 20 mg/kg tetrandrine treated rats, AST, ALT and T-Bil levels were lowered to 63.7% (P < 0.001), 58.1% (P < 0.001) and 79.9% (P < 0.01) compared with that of control fibrotic rats, respectively. In interferon-г treated fibrotic rats, AST, ALT and T-Bil levels were lowered to 74.4% (P < 0.05), 69.1% (P < 0.01) and 82.8% (P < 0.05) compared with that of control fibrotic rats, respectively.
| Group | AST (IU/L) | ALT (IU/L) | T-Bil (μmol/L) |
| Normal | 102 ± 24 | 34 ± 11 | 11.10 ± 1.29 |
| TAA (200 mg/kg) | 356 ± 70 | 198 ± 32 | 18.63 ± 2.50 |
| TAA + tetrandrine (5 mg/kg) | 266 ± 38a | 142 ± 29b | 15.70 ± 1.42a |
| TAA + tetrandrine (10 mg/kg) | 259 ± 45a | 139 ± 29b | 15.11 ± 2.26b |
| TAA + tetrandrine (20 mg/kg) | 227 ± 49d | 115 ± 30d | 14.89 ± 0.89b |
| TAA + interferon-г (5 × 104 U) | 265 ± 38a | 137 ± 25b | 15.43 ± 1.36a |
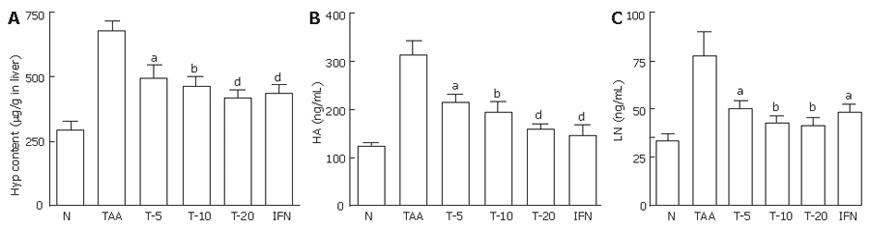
As shown in Figure 2A, liver contents of hydroxyproline increased to 229%, 4 wk after TAA-treatment (P < 0.001). Compared with the control fibrotic rats, in fibrotic rats treated with 5, 10 or 20 mg/kg tetrandrine, the hydroxyproline contents in the liver reduced to 73.3% (P < 0.05), 67.8% (P < 0.01) or 61.5% (P < 0.001), respectively. In interferon-г treated fibrotic rats, the hydroxyproline contents in the liver reduced to 64.6% (P < 0.001).
As shown in Figures 2B and 2C, serum contents of HA and LN increased to 251% and 233%, 4 wk after TAA treatment (P < 0.001). In the fibrotic rats treated with 5, 10, 20 mg/kg tetrandrine or interferon-г, HA content in the serum reduced to 68.2% (P < 0.05), 62.32% (P < 0.01), 50.73% or 47.09% (P < 0.001), respectively, compared with the control fibrotic rats. Compared with the control fibrotic rats, in fibrotic rats treated with 5, 10, 20 mg/kg tetrandrine or interferon-г, LN content in the serum reduced to 53.59% (P < 0.05), 55.09% (P < 0.01), 65.09% (P < 0.01) or 68.30% (P < 0.05), respectively.
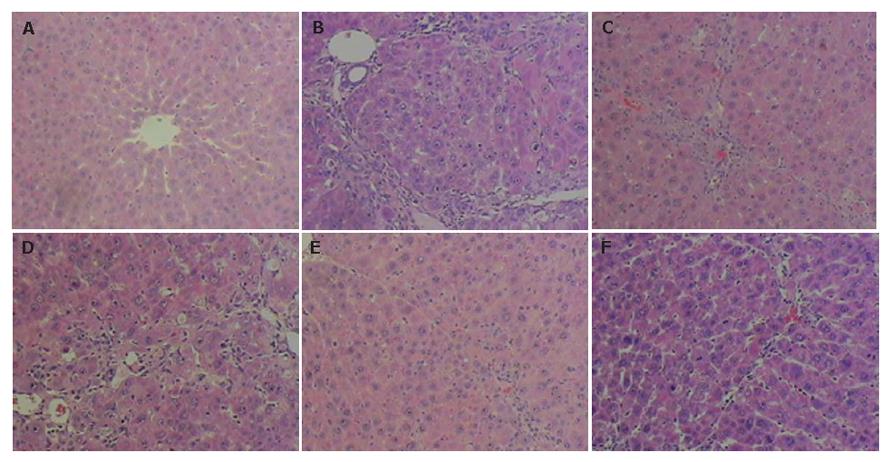
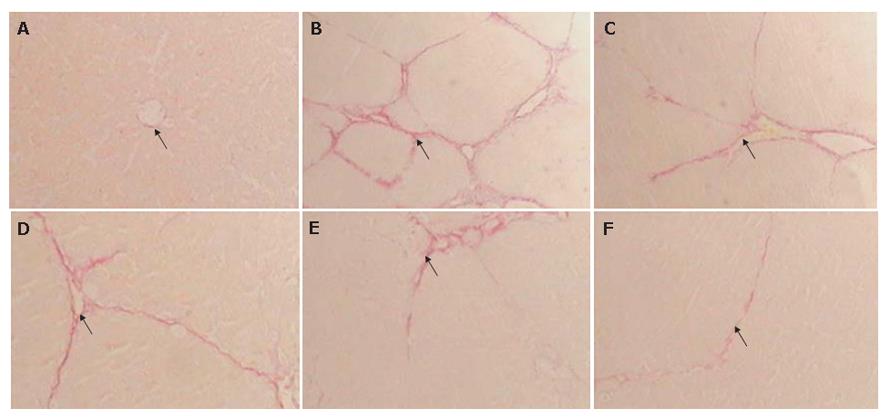
In control fibrotic group, the liver stained with HE and Sirius-red showed inflammation and an extensive accumulation of collagens (Figures 3B and 4B), fibrotic septa were increased and seen between areas of portal to portal and portal to central vein in some parts of liver lobules, and in some serious units pseudolobules presented. In tetrandrine and interferon-г treated fibrotic rats, there was a tendency towards less pronounced destruction of the liver architecture, compared with control fibrotic rat liver (Figure 3C-F, and Figure 4C-F).
As shown in Figures 3 and 4, TAA treatment for 4 wk resulted in liver injury, with loss of normal lobular architecture and a marked increase of collagen deposition throughout the liver. However, tetrandrine treatment resulted in reduced collagen deposition in the liver of TAA-treated rats.
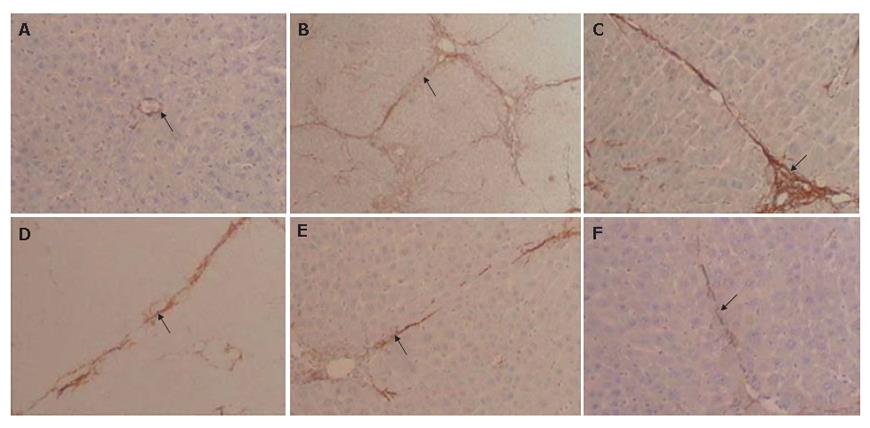
HSC activation was examined immunohistochemically by determining α-SMA-positive areas in injured rat livers treated with TAA. Considerable expression was detected in areas of centrilobular and periportal fibrotic bands in TAA treated rats (Figure 5B). In normal livers, collagen was observed only around the central and portal veins (Figure 5A). Activated HSCs were characterized by expression of α-SMA. In tetrandrine-treated or interferon-г groups, the expression of α-SMA was much lower than that in control fibrotic group and the distribution of α-SMA positive cells was similar to that of collagen in the liver (Figure 5C-F). In contrast, 20 mg/kg tetrandrine-treated rats notably reduced the positive areas of α-SMA in the livers of rats treated with TAA.
Thioacetamide (TAA) is a thiono-sulfur containing compound, undergoing an extensive metabolism to acetamide and TAA-S-oxide. Whereas acetamide is devoid of liver-necrotizing properties, TAA-S-oxide is further metabolized, at least in part, by cytochrome P-450 monooxygenases to other products, including a polar product that is thought to be the sulfene, TAA-S-dioxide, a highly reactive compound[21]. Therefore, TAA is a typical hepatotoxin and causes centrilobular necrosis by generation of ROS. Although TAA is known mainly to cause necrosis in the liver, it has been reported recently that TAA induced apoptosis in rat liver within a few hours after its administration[22]. The present study showed that a low dose of TAA induced apoptosis in the pericentral areas, whereas a high dose of TAA induced necrosis. Excessive ROS causes necrosis.
The mechanism by which tetrandrine inhibits rat hepatic stellate cell activation is not clear. Tetrandrine administration has been shown to block the calcium channels in various cell types[23]. Moreover, activated hepatic stellate cells have receptors for a number of vasoconstrictor substances such as endotheline, angiotensin II, vasopressin, thrombin and thromboxan A2[24-26]. Therefore, it is likely that the inhibitory effect of tetrandrine on hepatic stellate cell activation is, at least in part, through the blocking of calcium channels. However, further study is needed to understand the precise mechanism of tetrandrine on activated hepatic stellate cells.
Here we showed that tetrandrine is toxic against t-HSC/Cl-6 cells and antifibrotic. Previous studies demonstrated that tetrandrine induced apoptosis in t-HSC/Cl-6 cells, and activated rat hepatic stellate cells transformed by SV40. The morphologies of tetrandrine-treated cells revealed the typical features of apoptosis and these apoptotic phenomena were further confirmed by DNA laddering and annexin v-propidium iodide double staining[27]. Previous reports have demonstrated that the caspase family proteases play essential roles in the process of apoptosis[28]. Caspase-3, which has a short prodomain and is localized near nuclei, cleaves substrates at the downstream end of the cascade[29]. This study demonstrated that the activation of caspase-3 is involved in tetrandrine-induced cell death including apoptosis. Moreover, we have shown that tetrandrine is also effective in mediating HSC apoptosis in vivo after the development of fibrosis. It suggests that apoptosis of activated HSCs plays a key role in resolution of hepatic fibrosis.
Our data provide evidence that tetrandrine will effectively induce HSC apoptosis. In addition, we have demonstrated evidence that induction of apoptosis in HSCs enhances the resolution of experimental fibrosis. Taken together, these observations suggest that a strategy based on inducing HSC apoptosis may be an effective antifibrotic approach. The effects of tetrandrine at 20 mg/kg dose are better than the other groups. In conclusion, our results demonstrate that tetrandrine administration could prevent liver fibrosis and liver damage induced by TAA in rats. In this study, tetrandrine reduced the degree of hepatocellular injury as determined by lower serum levels of AST, ALT, T-bil and liver hydroxyproline, serum HA and LN, and also improved morphological structure of the fibrotic liver. Our study shows a direct inhibitory effect on rat HSC activation and an antifibrogenic potential of tetrandrine in fibrotic rats induced by TAA. Tetrandrine may have therapeutic effects in human liver fibrosis.
Fibrosis represents the response of the liver to diverse chronic insults such as chronic viral infection, alcohol, immunological attack, hereditary metal overload, parasitic diseases and toxic damage. It is accepted that HSCs play a central role in the development and resolution of liver fibrosis. Recently it was reported that during recovery from acute human and experimental liver injury, a number of activated HSCs undergo apoptosis, and there is a significant decrease in the extent of fibrosis within the same livers in association with this HSCs apoptosis.
Tetrandrine promotes HSC apoptosis in hepatic fibrosis. The study of its mechanism might lead to a new approach for fibrosis therapy. Further studies of the roles and regulation of HSC apoptosis and its possible mechanism are important for understanding the mechanism of resolution of liver fibrosis.
In this research, we investigated the effect of tetrandrine on the apoptotic death of rat HSCs in vitro and whether tetrandrine can prevent thioacetamide-induced fibrosis in vivo. The results showed that tetrandrine promotes the apoptosis of activated HSCs in vitro and prevent liver fibrosis and liver damage induced by thioacetamide in rats in vivo.
The results provide significant evidence illustrating the key feature of recovery from liver fibrosis is HSC apoptosis.
HSCs: hepatic stellate cells; Caspase: Caspases, closely associated with apoptosis, are aspartate-specific cysteine proteases and members of the interleukin-1b-converting enzyme family. The activation and function of caspases, involved in the delicate caspase-cascade system, are regulated by various kinds of molecules, such as the inhibitor of apoptosis protein, Bcl-2 family proteins, calpain, and Ca2+.
In this manuscript by Yin et al, the authors report effect of tetrandrine on the treatment of liver fibrosis-induced by thioacetamide in rats in vivo and in vitro. They employed assay of caspase activity to observe the enzymatic activity of caspases-3, a downstream caspase, occurred dose-dependently and investigated tetrandrine treatment as well as interferon-г significantly ameliorates the development of fibrosis as determined by lowered serum levels of AST, ALT, T-Bil and the levels of liver hydroxyproline, HA, LN and also improved histological findings. Their data may be critical for the study of the mechanism of resolution of rat liver fibrosis and shed new light on the liver fibrosis therapy.
S- Editor Wang J L- Editor Zhu LH E- Editor Lu W
| 1. | Gressner AM, Bachem MG. Cellular sources of noncollagenous matrix proteins: role of fat-storing cells in fibrogenesis. Semin Liver Dis. 1990;10:30-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 158] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Kayano K, Sakaida I, Uchida K, Okita K. Inhibitory effects of the herbal medicine Sho-saiko-to (TJ-9) on cell proliferation and procollagen gene expressions in cultured rat hepatic stellate cells. J Hepatol. 1998;29:642-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 886] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 4. | Carloni V, Pinzani M, Giusti S, Romanelli RG, Parola M, Bellomo G, Failli P, Hamilton AD, Sebti SM, Laffi G. Tyrosine phosphorylation of focal adhesion kinase by PDGF is dependent on ras in human hepatic stellate cells. Hepatology. 2000;31:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1596] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 6. | Iredale JP. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis. 2001;21:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 253] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 7. | Chojkier M, Brenner DA. Therapeutic strategies for hepatic fibrosis. Hepatology. 1988;8:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Park EJ, Ko G, Kim J, Sohn DH. Antifibrotic effects of a polysaccharide extracted from Ganoderma lucidum, glycyrrhizin, and pentoxifylline in rats with cirrhosis induced by biliary obstruction. Biol Pharm Bull. 1997;20:417-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Nan JX, Park EJ, Kim HJ, Ko G, Sohn DH. Antifibrotic effects of the methanol extract of Polygonum aviculare in fibrotic rats induced by bile duct ligation and scission. Biol Pharm Bull. 2000;23:240-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Nan JX, Park EJ, Kim YC, Ko G, Sohn DH. Scutellaria baicalensis inhibits liver fibrosis induced by bile duct ligation or carbon tetrachloride in rats. J Pharm Pharmacol. 2002;54:555-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Hui SC, Chan TY, Chen YY. Tetrandrine inhibits lipid peroxidation but lacks reactivity towards superoxide anion and hydrogen peroxide. Pharmacol Toxicol. 1996;78:200-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Reist RH, Dey RD, Durham JP, Rojanasakul Y, Castranova V. Inhibition of proliferative activity of pulmonary fibroblasts by tetrandrine. Toxicol Appl Pharmacol. 1993;122:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Liu BC, He YX, Miao Q, Wang HH, You BR. The effects of tetrandrine (TT) and polyvinylpyridine-N-oxide (PVNO) on gene expression of type I and type III collagens during experimental silicosis. Biomed Environ Sci. 1994;7:199-204. [PubMed] |
| 14. | Chen YJ, Tu ML, Kuo HC, Chang KH, Lai YL, Chung CH, Chen ML. Protective effect of tetrandrine on normal human mononuclear cells against ionizing irradiation. Biol Pharm Bull. 1997;20:1160-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Weinsberg F, Bickmeyer U, Wiegand H. Effects of tetrandrine on calcium channel currents of bovine chromaffin cells. Neuropharmacology. 1994;33:885-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Berlin JR. Spatiotemporal changes of Ca2+ during electrically evoked contractions in atrial and ventricular cells. Am J Physiol. 1995;269:H1165-H1170. [PubMed] |
| 17. | Nan JX, Park EJ, Kang HC, Park PH, Kim JY, Sohn DH. Anti-fibrotic effects of a hot-water extract from Salvia miltiorrhiza roots on liver fibrosis induced by biliary obstruction in rats. J Pharm Pharmacol. 2001;53:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Park PH, Nan JX, Park EJ, Kang HC, Kim JY, Ko G, Sohn DH. Effect of tetrandrine on experimental hepatic fibrosis induced by bile duct ligation and scission in rats. Pharmacol Toxicol. 2000;87:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Kim JY, Kim KM, Nan JX, Zhao YZ, Park PH, Lee SJ, Sohn DH. Induction of apoptosis by tanshinone I via cytochrome c release in activated hepatic stellate cells. Pharmacol Toxicol. 2003;92:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Jimenez W, Parés A, Caballería J, Heredia D, Bruguera M, Torres M, Rojkind M, Rodés J. Measurement of fibrosis in needle liver biopsies: evaluation of a colorimetric method. Hepatology. 1985;5:815-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Hunter AL, Holscher MA, Neal RA. Thioacetamide-induced hepatic necrosis. I. Involvement of the mixed-function oxidase enzyme system. J Pharmacol Exp Ther. 1977;200:439-448. [PubMed] |
| 22. | Ledda-Columbano GM, Coni P, Curto M, Giacomini L, Faa G, Oliverio S, Piacentini M, Columbano A. Induction of two different modes of cell death, apoptosis and necrosis, in rat liver after a single dose of thioacetamide. Am J Pathol. 1991;139:1099-1109. [PubMed] |
| 23. | Chen J, Wu Z, Chen S, Gong X, Zhong J, Zhang G. The effects of tetrandrine on the contractile function and microvascular permeability in the stunned myocardium of rats. Jpn J Physiol. 1999;49:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Kawada N, Tran-Thi TA, Klein H, Decker K. The contraction of hepatic stellate (Ito) cells stimulated with vasoactive substances. Possible involvement of endothelin 1 and nitric oxide in the regulation of the sinusoidal tonus. Eur J Biochem. 1993;213:815-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 268] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Bataller R, Nicolás JM, Ginès P, Esteve A, Nieves Görbig M, Garcia-Ramallo E, Pinzani M, Ros J, Jiménez W, Thomas AP. Arginine vasopressin induces contraction and stimulates growth of cultured human hepatic stellate cells. Gastroenterology. 1997;113:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Rockey D. The cellular pathogenesis of portal hypertension: stellate cell contractility, endothelin, and nitric oxide. Hepatology. 1997;25:2-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 191] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Zhao YZ, Kim JY, Park EJ, Lee SH, Woo SW, Ko G, Sohn DH. Tetrandrine induces apoptosis in hepatic stellate cells. Phytother Res. 2004;18:306-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Roy MK, Thalang VN, Trakoontivakorn G, Nakahara K. Mechanism of mahanine-induced apoptosis in human leukemia cells (HL-60). Biochem Pharmacol. 2004;67:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1304] [Article Influence: 56.7] [Reference Citation Analysis (0)] |