Published online Feb 21, 2007. doi: 10.3748/wjg.v13.i7.1103
Revised: December 17, 2006
Accepted: January 12, 2007
Published online: February 21, 2007
AIM: To evaluate the efficacy and mechanism of action of NCB-02, a standardized Curcumin preparation, against 2, 4-dinitrochlorobenzene (DNCB)-induced ulcerative colitis in rats.
METHODS: Ulcerative colitis was induced in male rats by sensitizing with topical application of DNCB in acetone for 14 d and intra-colonol challenge with DNCB on day 15. A separate group of animals with vehicle treatment in similar fashion served as control group. Colitis rats were divided into different groups and treated with NCB-02 at doses of 25, 50 and 100 mg/kg b.wt p.o. for 10 d. Sulfasalazine at a dose of 100 mg/kg b.wt for 10 d served as a reference group. On day 10 after respective assigned treatment, all the animals were euthanized and the length of the colon, weight of entire colon and distal 8 cm of the colon were recorded. The distal part of the colon was immediately observed under a stereomicroscope and the degree of damage was scored. Further distal 8 cm of the colon was subject to the determination of colonic myeloperoxidase (MPO), lipid peroxidation (LPO) and alkaline phosphatase (ALP) activities. A small piece of the sample from distal colon of each animal was fixed in 10% neutral buffered formalin and embedded in paraffin wax and sectioned for immunohistochemical examination of NFκ-B and iNOS expression.
RESULTS: NCB-02 showed a dose dependent protection against DNCB-induced alteration in colon length and weight. NCB-02 treatment also showed a dose dependent protection against the elevated levels of MPO, LPO and ALP, induced by DNCB. NCB-02 demonstrated a significant effect at a dose of 100 mg/kg b.wt., which was almost equipotent to 100 mg/kg b.wt. of sulfasalazine. Treatment with sulfasalazine and curcumin at a dose of 100 mg/kg b.wt. inhibited the DNCB-induced overexpression of NFκ-B and iNOS in the colon.
CONCLUSION: Curcumin treatment ameliorates colonic damage in DNCB-induced colitic rats, an effect associated with an improvement in intestinal oxidative stress and downregulation of colonic NFκ-B and iNOS expression.
- Citation: Venkataranganna M, Rafiq M, Gopumadhavan S, Peer G, Babu U, Mitra S. NCB-02 (standardized Curcumin preparation) protects dinitrochlorobenzene-induced colitis through down-regulation of NFκ-B and iNOS. World J Gastroenterol 2007; 13(7): 1103-1107
- URL: https://www.wjgnet.com/1007-9327/full/v13/i7/1103.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i7.1103
Inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis (UC), is a chronic and relapsing inflammatory disease caused by the inflammation and sores in the lining of large intestine and characterized clinically by recurrent episodes of bloody diarrhea, cramping, abdominal pain and histologically by mucosal inflammation and injury[1]. Conventional therapy for UC includes sulfasalazine and other 5-aminosalicylic acid (5-ASA) type of compounds, and in more persistent and/or severe cases, oral, rectal and parenteral corticosteroids and immunosuppressants are administered[2]. All of these have significant toxicities and are partly or completely ineffective in significant numbers of patients[3].
Several agents used in the management of IBD, such as corticosteroids, sulfasalazine and 5-ASA, have documented regulation of Nuclear Factor Kappa-B (NFκ-B) function[2]. Given the importance of inflammatory cell activation involved in the development of IBD, there is a need for a treatment modality against IBD that can block the inflammatory processes. There is substantial evidence for the involvement of oxidative stress and profound alterations in the biosynthesis of the labile free radical nitric oxide from L-arginine in the pathogenesis of colitis[4]. The use of medicinal plants or their active components has become an increasingly attractive approach for the treatment of UC. Curcumin, the active principle in turmeric, is a polyphenolic antioxidant and a natural yellow orange dye. Turmeric contains three curcumin analogues based on the number of hydroxyl groups present in the parent molecule. They are Curcumin (Curcumin I), demethoxy curcumin (Curcumin II) and bis-demethoxy curcumin (Curcumin III). Curcumin is a active constituent of Curcuma longa, whose anti-inflammatory properties are related in part to inhibition of the activities of the cyclooxygenase, lipoxygenase and NFκ-B in several cell systems[5-9]. Many experimental studies have demonstrated the important role of Curcumin in the attenuation of IBD and colonic cancer and it is also known to exhibit a variety of beneficial effects including antitumor, anti-HIV, antioxidant, anticataract development, septic shock, promotion of wound healing in normal and diabetic conditions, anti-asthmatic, anti-colitis, anti-fibrosis, reduction of mucosal damage, prevention of UV damage to skin, inhibition of development of cancers of the skin, stomach, colon, prostate, oral cavity and liver. Furthermore, Curcumin could also inhibit tumor metastases, pancreatitis, drug or alcohol-induced liver fibrosis, cystic fibrosis and Alzheimer's disease[10,11].
NCB-02 is a standardized extract of Curcuma longa containing 78% curcuminoids, 72% of which is Curcumin, 18.08% demethoxy curcumin and 9.42% bis-demethoxy Curcumin. This study was designed to evaluate its efficacy and mechanism of action against 2, 4-dinitrochloro-benzene (DNCB)-induced ulcerative colitis in rats.
Laboratory bred Wistar male rats weighing between 220-250 g were used for the experiments. The animals were housed and acclimatized to a constant temperature of 22 ± 3°C and were exposed to 12 h day and night cycle. The animals were fed with synthetic diet and water ad libitum.
Forty-eight rats were divided into six groups of eight animals each and, the nape hair was depleted. About 300 µL DNCB in acetone (20 g/L) was dropped to the nape of the rats once a day for 14 d. On the 15th day, animals of Groups II-VI were subject to intracolonol challenge of DNCB. Intra-colon challenge was done by infusing 0.25 mL of 0.1% DNCB in 50% alcohol into colon by a nylon catheter (3 mm in diameter), which was inserted into the colon at the site of 8 cm from the anus. GroupIserved as normal control and received 50% alcohol instead of DNCB. The animals were kept in Trendelenburg position for 1 min after DNCB administration and maintained in cages with free access to water and food.
Rats of Groups I and II served as normal and positive controls respectively and were administered with water (vehicle) at a dose of 10 mL/kg b.wt. p.o. and Group III rats with 100 mg/kg b.wt. p.o. of reference drug, sulfasalazine. Rats of groups IV-VI received 25, 50 and 100 mg/kg b.wt. p.o. of NCB-02, respectively. The treatment was carried out for 10 d, after challenge with DNCB. On d 10, after assigned treatment, all the animals were euthanized by exsanguinations. The entire length of the colon starting from the ceacal end was excised, opened and gently rinsed with ice-cold saline. The colon was kept flat with the mucosal surface upward on a plate prechilled to 4°C. The length of the colon, weight of entire colon and distal 8 cm of the colon were recorded. The distal part of the colon was immediately observed under stereomicroscope to note any visible damage. The degree of damage was scored macroscopically on a 0-5 scale by independent observers[13]. Further distal 8 cm of the colon was subject to the determination of colonic myeloperoxidase[14], lipid peroxides[15] and ALP activity. A small piece of the sample from distal colon of each animal was fixed in 10% neutral buffered formalin and embedded in paraffin wax and sectioned for immunohistochemical examination[16].
MPO is an enzyme found in cells of myeloid origin, and has been used extensively as a biochemical marker of granulocyte (mainly neutrophil) infiltration into gastrointestinal tissues[14]. Samples of distal colon were homogenized in 10 mmol/L potassium phosphate buffer, pH 7.0 containing 0.5% hexadecyltrimethylammonium bromide and centrifuged for 30 min at 20 000 ×g at 4°C. An aliquot of the supernatant was then allowed to react with a solution of 1.6 mmol/L O-dianisidine and 0.1 mmol/L H2O2. The rate of change in absorbance was measured spectrophotometrically at 650 nm. One unit of myeloperoxidase activity was defined as degrading 1 mmol of H2O2 per min at 37°C and was expressed as units per milligram of tissue sampled (U/mg tissue).
The tissue was homogenized at a concentration of 10% w/v in 0.15 mol/L potassium chloride using a glass homogenizer. The homogenate was centrifuged at 800 ×g and the supernatants were used for the estimation of lipid peroxides[15] and alkaline phosphatase (ALP) using Boheringer Mannheim kit.
Colon sections were deparaffinized in xylene, for 2-5 min and dehydrated with 100% ethanol for 2-3 min followed with 95% ethanol for 1 min, then rinsed in distilled water. Tissue sections were incubated with primary antibody at appropriate dilution in phosphate buffered saline (PBS) for 1 h at room temperature, then incubated in biotinylated secondary antibody in PBS for 1 h at room temperature. After rinsing in PBS for 3 changes of 2 min each, sections were incubated in freshly prepared peroxidase substrate solution for 10 min at room temperature and counterstained with weak haematoxylin for 10 min after rinsing with PBS.
The values were expressed as mean ± SE. The results were analyzed statistically using one-way ANOVA followed by Dunnet’s multiple comparison test using GraphPad Prism software package (Version 4.0) to find the level of significance. The minimum level of significance is fixed at P < 0.05.
Rats sensitized and challenged with DNCB showed ulcers with severe macroscopic inflammation in the colon as assessed by the colonic damage score. Colon length was significantly reduced with a significant increase in total and distal colon weight in colitic rats as compared with non-colitic control rats. There was also a significant increase in colonic MPO, LPO and ALP activity as compared with non-colitic rats (Table 1). Treatment with NCB-02 showed a dose dependent protection against DNCB-induced colonic damage as indicated by normalization of colon length, reduction in colon weight (total and distal) and decrease in the levels biochemical markers such as MPO, LPO and ALP. NCB-02 at a dose of 100 mg/kg b.wt. p.o. showed a significant effect on various parameters (Table 1). Reference drug sulfasalazine presented with a significant reversal of DNCB-induced alterations at a dose of 100 mg/kg b.wt. p.o. (Table 1).
| Ulcer index | Colon length | Colon Wt.(Total) (gm) | Distal Colon wt. (last 8 cm) (gm) | MPO Units/gmof tissue | LPO nmol/L per100 mg | ALP IU/100 mg | |
| Control | 0.00 ± 0.00 | 19.88 ± 0.41 | 1.46 ± 0.05 | 0.52 ± 0.02 | 2.98 ± 0.42 | 145.75 ± 13.78 | 26.63 ± 3.45 |
| Positive control | 4.50 ± 0.25b | 15.75 ± 0.39b | 2.18 ± 0.13b | 1.33 ± 0.10b | 5.63 ± 0.46b | 296.23 ± 23.98b | 252.13 ± 33.69d |
| Sulfasalazine (100 mg/kg) | 2.75 ± 0.39f | 18.13 ± 0.37h | 1.76 ± 0.05j | 0.84 ± 0.05h | 3.60 ± 0.29j | 211.54 ± 32.23j | 136.38 ± 17.69j |
| NCB-02 (25mg/kg) | 3.75 ± 0.23 | 15.13 ± 0.37 | 1.98 ± 0.14 | 1.22 ± 0.13 | 4.27 ± 0.45 | 259.38 ± 28.71 | 232.38 ± 33.79 |
| NCB-02 (50mg/kg) | 3.00 ± 0.18j | 16.50 ± 0.31 | 1.88 ± 0.15 | 1.09 ± 0.09 | 3.90 ± 0.32 | 205.69 ± 22.09j | 186.88 ± 24.86 |
| NCB-02 (100mg/kg) | 2.63 ± 0.25f | 18.13 ± 0.45h | 1.69 ± 0.07j | 0.90 ± 0.06j | 3.73 ± 0.30j | 174.84 ± 13.06h | 153.13 ± 20.23j |
Sulfasalazine treatment resulted in significant protection with the mean ulcer score of 2.75 ± 0.39 and NCB-02 had a dose dependent protection at 25, 50 and 100 mg/kg b.wt. with ulcer scores of 3.75 ± 0.23, 3.00 ± 0.18 and 2.63 ± 0.25 (P < 0.001), respectively (Table 1).
Myeloperoxidase activity is an established marker for inflammatory cell (mainly neutrophils) infiltration in rodent models of colitis, and was thus examined. MPO activity was significantly increased in colitis rats as compared with the control. NCB-02 treatment inhibited DNCB-induced MPO activity in a dose dependent manner. Both sulfasalazine and NCB-02 at a dose of 100 mg/kg b.wt. significantly protected DNCB-induced elevation of MPO activity (Table 1).
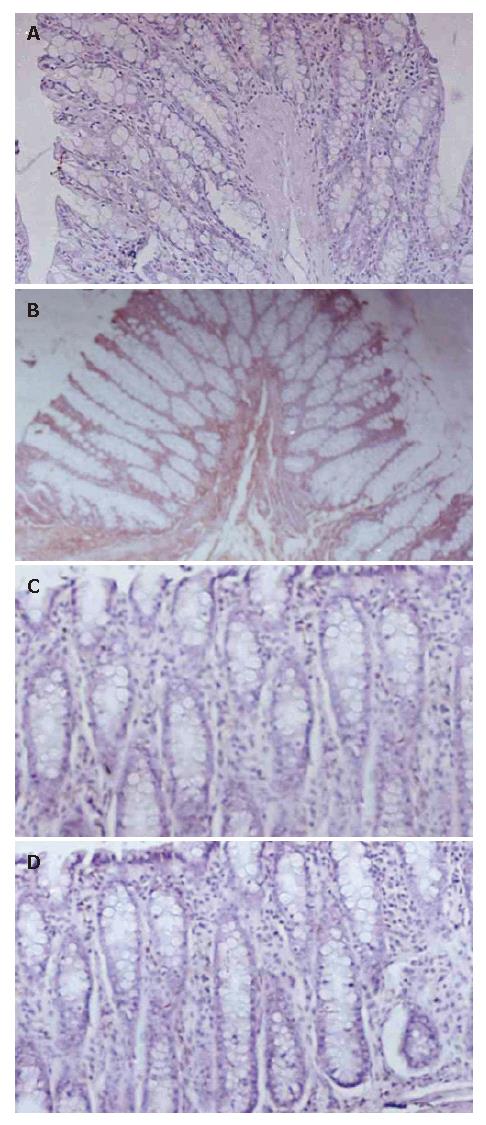
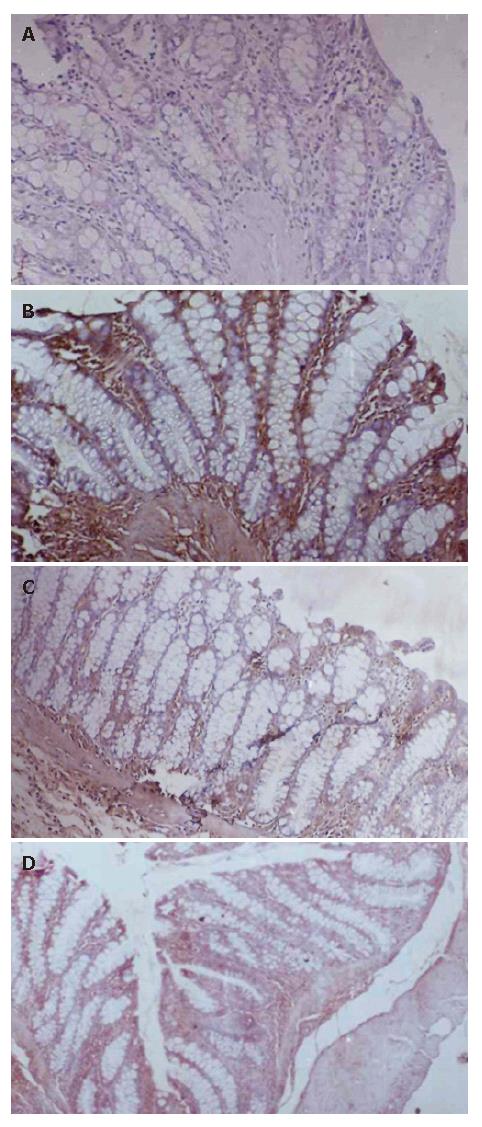
High level expression of iNOS and NFκ-B were observed in colitis rats, which is revealed by immunohisto-chemistry (Figures 1 and 2). This immunohistochemistry for NFκ-B and iNos expression revealed that NCB-02 at a dose 100 mg/kg b.wt. inhibited the DNCB-induced expression of these pro-inflammatory mediators of ulcerative colitis.
From the various parameters evaluated it was observed that 100 mg/kg b.wt. of NCB-02 was almost equipotent to sulfasalazine at 100 mg/kg b.wt.
In the present investigation, the beneficial effects observed with NCB-02 (Standardized Curcumin preparation) treatment were assessed based on the improvement of colon weight and length, and histologically by preservation of the colon architecture in comparison with the rats from the non-treated colitic group. The biochemical assays, such as MPO and ALP, performed in the colonic specimens confirmed the anti-inflammatory effect exerted by Curcumin at a dose of 100 mg/kg, since it was associated with significant reduction in MPO and ALP activities. MPO activity has been widely used to detect and follow intestinal inflammation, and a reduction in the activity of this enzyme can be interpreted as a manifestation of the anti-inflammatory activity of a given compound. Alkaline phosphatase activity is considered as one of the sensitive markers of inflammation in the intestine, as this enzyme activity is invariably augmented in these experimental conditions of colitis. The results obtained in this study confirm the intestinal anti-inflammatory effect previously demonstrated for Curcumin[9,17].
Earlier studies provided evidence of significantly elevated activation of NFκ-B in ulcerative colitis and Crohn’s disease[18,19]. Several therapeutic agents with NFκ-B inhibitory activity, such as sulfasalazine, mesalamine, and corticosteroids have been used for the treatment of IBD. They have sundry defects such as steroid dependence and steroid resistance, decreasing glucose tolerance, hepatotoxicity and pancreatitis[2]. Now, more potent and selective treatment strategies for IBD aim at preventing NFκ-B activation in mucosal macrophages and T lymphocytes.
According to the well-described role of NFκ-B in inflammatory regulation and iNOS expression, the degree of NFκ-B expression was determined in the colonic tissue samples from animals subjected to DNCB sensitization and challenge. The inflammatory status induced by DNCB was associated with increased colonic NFκ-B expression when compared to normal tissues. Curcumin treatment resulted in inhibition of NFκ-B expression and similar response was observed with sulfasalazine treatment.
Nitric oxide is one of the important pro-inflammatory mediators, which plays a key role in the pathogenesis of IBD. In our study, we evaluated the effect of Curcumin on colonic iNOS activity in the DNCB-induced model of experimental colitis. DNCB administration increased colonic iNOS activity expression in rats, as detected by immunohistochemistry (Figure 2B). The intestinal anti-inflammatory effect exerted by NCB-02, was associated with a reduction of iNOS expression (Figure 2D) when compared with DNCB control animals.
Myeloperoxidase is an enzyme found in cells of myeloid origin, and has been used extensively as a biochemical marker of granulocyte (mainly neutrophil) infiltration into gastrointestinal tissues. Our study showed that DNCB raised the levels of colonic MPO, which was ameliorated in NCB-02 and sulfasalazine treated groups.
In addition, our study gives some evidence about the mechanisms involved in the intestinal anti-inflammatory effect of Curcumin. One of the mechanisms could be its inhibition of free radical generation and antioxidant properties, which is evident from several earlier observations[9,10]. This activity may play a crucial role in the intestinal anti-inflammatory effect of the Curcumin, because intense oxidative insult is a common feature in human IBD and in the different experimental models of rat colitis, such as the trinitrobenzene sulfonic acid (TNBS) and the DNCB models in rats, and is an important mechanism for tissue damage during chronic intestinal inflammation[18].
In the last decade, it became increasingly clear that NO overproduction by iNOS is deleterious to intestinal function, which contributes significantly to gastrointestinal immunopathology in the chronic inflammatory events in IBD[16,18]. The important role attributed to NO in these intestinal conditions prompted us to study whether the beneficial effects of Curcumin on DNCB-induced colitis could be related to an effect on colonic NO production. The results in this study reveal that colonic inflammation is associated with a higher colonic iNOS expression, as evidenced by immunohistochemistry. Treatment of colitic rats with NCB-02 effectively inhibited the upregulated colonic iNOS expression.
The present study revealed that oral treatment of NCB-02 in colitic rats significantly inhibited the NFκ-B pathway, which is reported to be activated as a consequence of the intestinal inflammatory process induced by DNCB. The molecular mechanism involved in the suppressive effects of Curcumin on NFκ-B could be due to the inhibition of NFκ-B by acting as antioxidants, since NFκ-B is a redox-sensitive transcription factor and activated by oxidative stress in the inflamed intestinal mucosa via blocking the phosphorylation and degradation of IkB protein, as previously reported in vitro and in vivo[7,19].
In conclusion, Curcumin treatment ameliorates colonic damage in DNCB-induced colitic rats, an effect associated with an improvement in intestinal oxidative stress and a downregulation in colonic iNOS and NFκ-B expression. Therefore, Curcumin (NCB-02) may hold promise for the treatment of inflammatory bowel disease.
S- Editor Liu Y L- Editor Ma JY E- Editor Ma WH
| 1. | Furrie E, Macfarlane S, Cummings JH, Macfarlane GT. Systemic antibodies towards mucosal bacteria in ulcerative colitis and Crohn's disease differentially activate the innate immune response. Gut. 2004;53:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Nikolaus S, Fölscn U, Schreiber S. Immunopharmacology of 5-aminosalicylic acid and of glucocorticoids in the therapy of inflammatory bowel disease. Hepatogastroenterology. 2000;47:71-82. [PubMed] |
| 3. | Farrell RJ, Kelleher D. Glucocorticoid resistance in inflammatory bowel disease. J Endocrinol. 2003;178:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Barbosa DS, Cecchini R, El Kadri MZ, Rodríguez MA, Burini RC, Dichi I. Decreased oxidative stress in patients with ulcerative colitis supplemented with fish oil omega-3 fatty acids. Nutrition. 2003;19:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Han SS, Keum YS, Seo HJ, Surh YJ. Curcumin suppresses activation of NF-kappaB and AP-1 induced by phorbol ester in cultured human promyelocytic leukemia cells. J Biochem Mol Biol. 2002;35:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Pan MH, Lin-Shiau SY, Lin JK. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IkappaB kinase and NFkappaB activation in macrophages. Biochem Pharmacol. 2000;60:1665-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 262] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 7. | Zhang F, Altorki NK, Mestre JR, Subbaramaiah K, Dannenberg AJ. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis. 1999;20:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 201] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, Sartor RB. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J Immunol. 1999;163:3474-3483. [PubMed] |
| 9. | Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem. 1995;270:24995-25000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 984] [Cited by in RCA: 984] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 10. | Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995;94:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 690] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 11. | Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363-398. [PubMed] |
| 12. | Jiang XL, Cui HF. A new chronic ulcerative colitis model produced by combined methods in rats. World J Gastroenterol. 2000;6:742-746. [PubMed] |
| 13. | Fan H, Qiu MY, Mei JJ, Shen GX, Liu SL, Chen R. Effects of four regulating-intestine prescriptions on pathology and ultrastructure of colon tissue in rats with ulcerative colitis. World J Gastroenterol. 2005;11:4800-4806. [PubMed] |
| 14. | Perner A, Andresen L, Normark M, Fischer-Hansen B, Sørensen S, Eugen-Olsen J, Rask-Madsen J. Expression of nitric oxide synthases and effects of L-arginine and L-NMMA on nitric oxide production and fluid transport in collagenous colitis. Gut. 2001;49:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Mitra SK, Venkataranganna MV, Sundaram R, Gopumadhavan S. Antioxidant activity of AO-8, a herbal formulation in vitro and in vivo experimental models. Phytother Res. 1999;13:300-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Dong WG, Liu SP, Yu BP, Wu DF, Luo HS, Yu JP. Ameliorative effects of sodium ferulate on experimental colitis and their mechanisms in rats. World J Gastroenterol. 2003;9:2533-2538. [PubMed] |
| 17. | Ukil A, Maity S, Karmakar S, Datta N, Vedasiromoni JR, Das PK. Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br J Pharmacol. 2003;139:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 178] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 18. | Monteleone G, Mann J, Monteleone I, Vavassori P, Bremner R, Fantini M, Del Vecchio Blanco G, Tersigni R, Alessandroni L, Mann D. A failure of transforming growth factor-beta1 negative regulation maintains sustained NF-kappaB activation in gut inflammation. J Biol Chem. 2004;279:3925-3932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Jian YT, Mai GF, Wang JD, Zhang YL, Luo RC, Fang YX. Preventive and therapeutic effects of NF-kappaB inhibitor curcumin in rats colitis induced by trinitrobenzene sulfonic acid. World J Gastroenterol. 2005;11:1747-1752. [PubMed] |