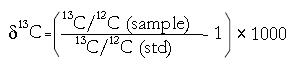Published online Feb 21, 2007. doi: 10.3748/wjg.v13.i7.1079
Revised: December 1, 2006
Accepted: January 22, 2007
Published online: February 21, 2007
AIM: To develop a new formulation with hydroxy propyl methyl cellulose and Shellac coating for extended and selective delivery of butyrate in the ileo-caecal region and colon.
METHODS: One-gram sodium butyrate coated tablets containing 13C-butyrate were orally administered to 12 healthy subjects and 12 Crohn’s disease patients and the rate of 13C-butyrate absorption was evaluated by 13CO2 breath test analysis for eight hours. Tauroursodeoxycholic acid (500 mg) was co-administered as a biomarker of oro-ileal transit time to determine also the site of release and absorption of butyrate by the time of its serum maximum concentration.
RESULTS: The coated formulation delayed the 13C-butyrate release by 2-3 h with respect to the uncoated tablets. Sodium butyrate was delivered in the intestine of all subjects and a more variable transit time was found in Crohn’s disease patients than in healthy subjects. The variability of the peak 13CO2 in the kinetic release of butyrate was explained by the inter-subject variability in transit time. However, the coating chosen ensured an efficient release of the active compound even in patients with a short transit time.
CONCLUSION: Simultaneous evaluation of breath 13CO2 and tauroursodeoxycholic acid concentration-time curves has shown that the new oral formulation consistently releases sodium butyrate in the ileo-cecal region and colon both in healthy subjects and Crohn’s disease patients with variable intestinal transit time. This formulation may be of therapeutic value in inflammatory bowel disease patients due to the appropriate release of the active compound.
- Citation: Roda A, Simoni P, Magliulo M, Nanni P, Baraldini M, Roda G, Roda E. A new oral formulation for the release of sodium butyrate in the ileo-cecal region and colon. World J Gastroenterol 2007; 13(7): 1079-1084
- URL: https://www.wjgnet.com/1007-9327/full/v13/i7/1079.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i7.1079
Butyric acid, a short chain fatty acid, is the main colonic bacterial product of non-starch polysaccharides[1]. Impaired butyric acid metabolism (usually as butyrate species) has been implicated in the development of ulcerative colitis (UC)[2] but there are conflicting data regarding its role in the pathogenesis of UC[3-11]. Large bowel mucosa biopsy specimens from quiescent UC patients have also shown reduced oxidation of butyrate but not of glucose and glutamine, the two other major fuel sources for colonic epithelium[3]. In vitro studies conducted in terminal ileal mucosa biopsy specimens from UC patients support this concept[4]. A study on colonic sodium butyrate (NaB) metabolism using 14C-butyrate rectal instillation and 14CO2-breath test in patients affected by extensive UC showed a reduction in NaB oxidation, which returned to normal on remission[10]. The authors concluded that while impaired NaB metabolism is unlikely to be a primary cause of UC, impairment of short chain fatty acid metabolism by coloncytes may be a pathogenic factor. Another study indicated that in quiescent UC patients, the rate of NaB metabolism is not impaired[11].
It has recently been demonstrated that NaB exhibits anti-inflammatory properties as documented by a strong inhibition of interleukin (IL)-12 production by suppressing both IL-12p35 and IL-12p40 mRNA accumulation, and enhances IL-10 secretion in Staphylococcus aureus cell-stimulated human monocytes[12]. Investigation of the effects of NaB on some G1 phase-related proteins in a colon carcinoma cell line (HT29) has revealed another potential pharmacological property, since NaB is able to reduce cyclin D1 and p53 level in a dose-dependent fashion and to suppress cell growth[13]. Thus, the lack of NaB in diets poor in carbohydrates could lead to clinically relevant functional alterations. In vivo, the growth inhibitory effects of NaB on colon cancer cells appear to be somewhat less marked[14,15].
Some clinical applications of NaB treatment have already been successfully evaluated. NaB enemas seem to provide an effective treatment for acute radiation proctitis, accelerating the healing process[16,17]. Oral co-administration of NaB and mesalazine in patients with active UC seems to improve the efficacy of mesalazine monotherapy[18,19].
These considerations prompted us to develop a new oral formulation in which NaB is released in the terminal ileum and colon, the target of its potential pharmacological activity[19].
The main problem in the development of controlled release formulation is to extrapolate in vitro release data to those achieved during in vivo studies. This is particularly crucial for drugs used in patients with gastrointestinal diseases in which the intestinal transit time shows a high variability.
The colonic target release of enteric coated butyrate requires an optimized coating able to prevent an early release (duodenum-jejunum-ileum) resulting in an absorption and metabolism of butyrate before reaching the colon. The coating is expected to release NaB from the ileo-caecal region as a result of a variation of intestinal pH but a delayed release delivery kinetic would result in a loss of a relatively high amount of butyrate in the stools.
Tuleu et al[20] have developed pellets for colonic delivery of NaB via oral route. When they are administered to rats a large amount is lost in the caecum for coating dissolution problems.
Optimization of the tablets is therefore crucial to determine in the healthy subjects and patients with Inflammatory bowel disease (IBD) the exact delivery site of butyrate in the intestine thus reducing false negative data in term of efficacy due to a poor delivery of the active compound in the target area.
Sodium butyrate tablets (1 g) coated with hydroxyl propyl methylcellulose (HPMC) and natural polymer Shellac have been developed and optimized by conventional in vitro studies. In the present study the optimal coating thickness was optimized in vivo by evaluating the release of the active ingredient by the kinetics of 13CO2 excretion in the breath of healthy subjects and Crohn’s disease patients after oral administration of 13C-labeled NaB included in the 1 g tablets. The rate of 13CO2 production is the result of 13C-NaB intestinal absorption and metabolism that was evaluated by the rate of production of 13CO2 in exhaled breath over 8 h. The 13C/12C isotope ratio was measured by isotope ratio mass spectrometry (IRMS). The intestinal transit time was evaluated by simultaneous co-administration of tauroursodeoxycholic acid (TUDCA).
TUDCA was selected as a biomarker of oro-ileal transit time since it is absorbed actively only in the ileum, the time of its peak serum level reflects the oro-ileal transit time. A sensitive and specific enzyme immunoassay was performed to evaluate serum TUDCA concentration at various time intervals after administration as previously described[21].
To evaluate the kinetics of the release of NaB from the tablets, we compared the time of maximum 13CO2 excretion with that of peak serum TUDCA concentration within individual subjects in order to overcome the high variability in intestinal transit time.
Plain and coated tablets containing hydroxyl propyl methylcellulose and shellac with a pH dependent extended release coating were used in the study. Both formulations contained 1 g of NaB and three batches of tablets containg 5%, 10% and 20% w/w of 13C-NaB (CIL, Andover MA, USA) were prepared.
The difference between the two tablets was the internal pre-coating of HPMC and external coating of shellac, which is resistant to desgregation up to pH 7. All other excipients of the formulations were identical. The pre-coating of HPMC was made to avoid that basic characteristics of the active ingredient in the tablet nucleus would induce an early dissolution of shellac which is resistant to acid pH and soluble to basic pH. In order to optimize the release of NaB in the colon, different thickness films of shellac (50, 80 and 120 μm) were studied. Coated tablets (Sobutir) were supplied by Promefarm srl, Milan, Italy. The different formulations were then administered to the same subjects in order to verify simultaneously the kinetic of NaB release in relation to the oro-ileal transit time evaluated by the time of the serum TUDCA peak and select the formulation that would be admitted to the complete study.
The study was carried out in 12 healthy subjects (6 males and 6 females, median age 42 years, range 18-60 years) and 12 Crohn’s disease patients (9 females and 4 males, median age 40 years, range 18-65 years) with a relatively mild activity index ranging from 320 ≥ C.D.A.I. ≥ 220[22]. To avoid any difference in the transit time, we studied only patients with the same level of activity index. In addition, the patients studied had no pure ileal or ileo-colonic involvement and they did not receive steroid or immunosuppressive treatment. All patients during the study presented diarrhoea. Before this final controlled study volunteers received the different formulations under development and between the different studies a washout period of one week was used. Thirty minutes before the study, 2 mL of blood was collected for the baseline values, as were two separate samples of breath in a 10 mL capped glass tube. After administration of the 1 g 13C-NaB tablet and of 500 mg TUDCA as gelatine capsules (Tudcabil®, Pharmacia Upjohn, Milan, Italy), the subjects received a standard liquid test meal of 375 Kcal, containing 17 g of fat, 10.4 g of protein and 10 g of carbohydrates. Breath and blood samples were collected at 30 min intervals over an 8-h period (unless otherwise specified). The oro-ileal transit time was defined as the time interval between administration of TUDCA and the peak serum TUDCA (Cmax) time[21]. The time of the maximum 13CO2 breath excretion was used to represent the time of release and absorption of sodium butyrate. The study approved by the Ethical Committee of University of Bologna was conducted according to the institutional guidelines.
13CO2 breath test:13CO2 in each of the duplicate breath samples was analyzed by continuous flow isotope ratio mass spectrometry (IRMS; ANCA, PDZ Europa Ltd, Cheshire U.K.). The results were expressed as δ13C that was calculated from:
Math 1
where 13C/12C (std) is the Pee Dee Belemnite (PDB) reference standard 13C/12C ratio, the final value was expressed as a milli percentage (δ13C%).
Serum TUDCA levels were determined by a specific chemiluminescent enzyme immunoassay previously developed and optimized in our laboratory[21]. The method is a solid-phase competitive format with a TUDCA-specific polyclonal antibody immobilized on 96 wells black polystyrene microtiter plates. A horseradish peroxidase (HRP)-UDCA conjugate was synthesized, purified, properly characterized, and used as enzymatic tracer.
For the TUDCA assay 100 μL of the sample (serum diluted 1/50, v/v with assay buffer: 0.05 mol/L phosphate/EDTA buffer, pH 7.4, containing 1 g/L bovine serum albumin) or of six standard TUDCA solutions with a concentration ranging from 0.01 to 1000 nmol/L was incubated in the 96-well microtiter plates coated with the antibody for 1 h at 37°C with 100 μL of the properly diluted HRP-UDCA tracer. After washed with assay buffer, 100 μL of the chemiluminescent substrate (H2O2/luminol/enhancer SuperSignal ELISA Pico, Pierce, II, USA) was added and the light signal was measured using a PMT based luminometer microtiter reader (Luminoskan Ascent, Thermo Electron Corporation MA, USA) .
TUDCA concentrations were determined by a plot of chemiluminescent (CL) signal vs the log of concentration and the best data fit was obtained by linear regression of the six point standards. Serum TUDCA was expressed as nmol/L of serum.
The 13C-NaB dose to include in the 1 g NaB tablet was standardized by quantifying the amount of label to produce a breath CO2 enrichment of the 13C-isotope that could be accurately evaluated with respect to the baseline value representing its natural abundance.
During the course of three separate weeks, three 1-g NaB tablets containing 5%, 10% and 20% w/w of 13C-NaB respectively, were administered to six healthy subjects. The excretion rate of 13CO2 in breath was measured and the maximum value was calculated. The dose of 100 mg of the label was chosen giving a δ13C% of 15.3 that was much higher than the baseline value of -28%. This wide cut off could allow the accurate evaluation of not only the maximum excretion of 13CO2 but also small variations during the 8-h study period and eventually low 13CO2 excretion resulting in patients with impaired metabolism of NaB. The coating thickness was optimized according to the kinetics of the 13CO2 excretion accounting for the release and absorption of butyrate from the tablet. The results obtained with 50, 80 and 120 μm thickness coating suggested that the coating size of 80 μm was the most adequate to prevent a too early release and absorption of NaB as occurred with the 50 μm thickness and to ensure complete release of the active ingredient in a time comparable with the oro-ileal transit time.
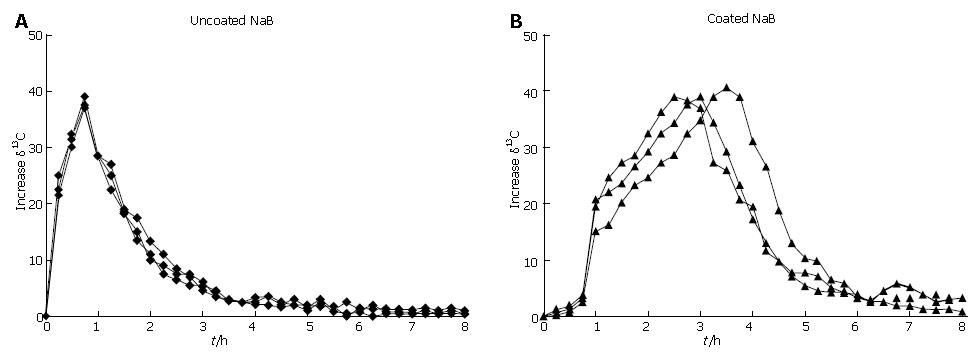
The NaB tablet containing 10% w/w of 13C-NaB and 80 μm-thick coating was used to evaluate the final performance of the formulation. The intra-subject variability was evaluated by administering either coated or uncoated NaB formulations three times to the same subject in separate experiments and monitoring the excretion of 13CO2 at 15 min time intervals (Figure 1). In the uncoated tablet, the intra-subject variability was very low and the curves were almost super impossible. The maximum excretion was achieved 45 min after administration of the dose and the absorption started just in the first 15 min. A slightly higher variability was observed when the coated formulation was administered as a result of an intra-subject variability in gastric emptying and overall gastrointestinal transit. The mean peak 13CO2 excretion time of the coated tablets was 180 min (range 150-225 min), showing the extended release of NaB caused by the shellac coating.
Chemiluminescent enzyme immunoassay fulfilling all the standard requirements of accuracy and precision was used. The intra and inter studies showed that the coefficient of variation was always below 9%. The limit of quantification of 10 nmol/L allowed direct analysis of TUDCA in a 20-fold diluted serum sample.
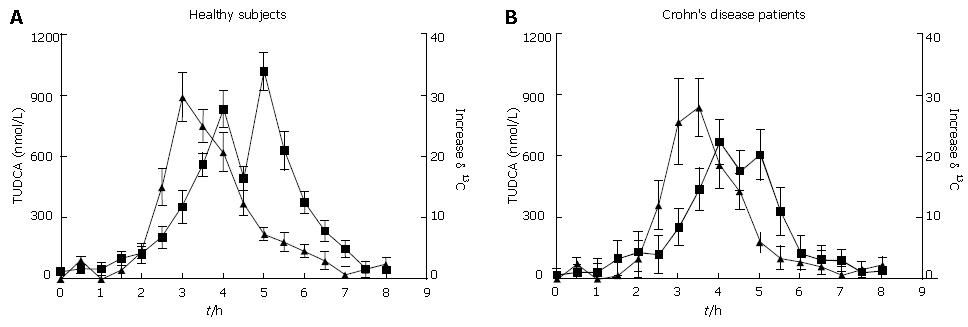
The mean serum TUDCA profiles together with the kinetics of 13CO2 excretion obtained in the healthy subjects and Crohn’s disease patients are shown in Figure 2. The serum TUDCA profile was characterized by two peaks. The first peak was reached 180-200 min after TUDCA administration and represented the oro-ileal transit time, the second peak was observed after 300-400 min as a result of the enterohepatic circulation of the absorbed TUDCA.
In the healthy subjects peak of serum TUDCA concentration was achieved at a median of 4 h (range 3-5 h) after NaB administration. The mean peak 13CO2 excretion time occurred slightly earlier at 3.5 h and the range of variability was similar to that of TUDCA. A similar behavior was observed in Crohn’s disease patients with a higher variability due to variation in the intestinal transit time.
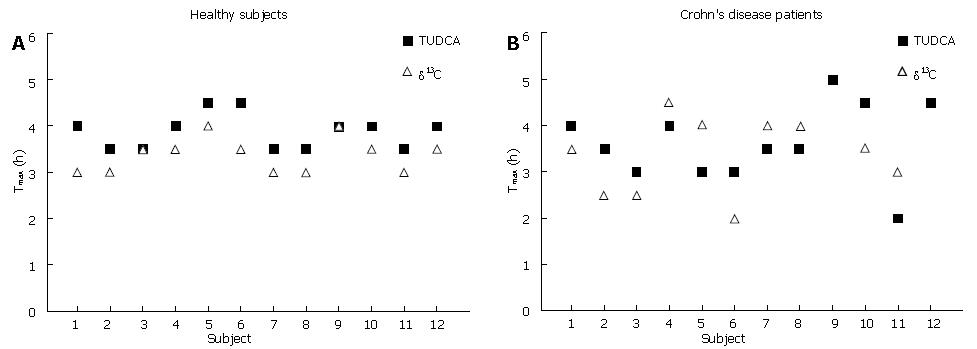
Top panel and bottom panel are shown in Figure 3. The Tmax was obtained in control subjects and Crohn’s disease patients using the serum TUDCA and 13CO2 breath tests. No subject and Crohn’s disease patient had an earlier release of 13CO2 after administration of the coated tablets with respect to the Tmax of TUDCA. Similarly no subject and patient missed to give a peak of 13CO2 excretion or reported the loss of intact tablets in stools.
The ileal release was defined (a priori) as the 13CO2 peak occurring within 30 min from the time of TUDCA peak whereas colonic release was considered as the 13CO2 peak occurring after this interval.
The temporal correspondence between the maximum 13CO2 excretion times, with the TUDCA time at the Cmax representing the biomarker oro-ileal transit time even if occurring at different times, usually slightly earlier suggested that the active ingredient was still delivered in the colon in high concentration and therefore we expected that it would exert its activity in IBD patients.
In all the studied subjects, NaB was efficiently released by the coated tablet, suggesting that the formulation developed could be used in these subjects to prevent an early absorption and deliver a large amount of NaB in the colon just starting from the terminal ileum as shown by the kinetic profile of 13CO2 and serum TUDCA.
The present study was designed to evaluate a new NaB formulation able to deliver the active ingredients of 1 g tablets coated with shellac into the colon by oral administration. The coating delayed the release of the active ingredients by two-three hours with respect to the uncoated formulation, thus a large amount of NaB could reach the colon, as demonstrated by the temporal similarity between the profiles of 13CO2 breath test and serum TUDCA concentration. In fact, TUDCA is absorbed by an active carrier-mediated mechanism only in the ileum[23,24], and previous studies comparing the TUDCA serum levels after its oral administration with other markers of intestinal transit-time such as sulfasalazine showed that this method provides a valid and practical means of assessment of the oro-ileal transit-time[21]. Comparison of the peak times of 13CO2 excretion and serum TUDCA concentration could confirm the efficacy of shellac coating in delivering NaB to the colon independently of intestinal transit-time variability. The dissolution of Shellac coating at pH 7 and its thickness driving the kinetics of the dissolution process has been well optimized and a large amount of NaB which starts to be delivered after two hours reaches the intact ileo-cecal region as shown by the kinetics of TUDCA intestinal absorption.
It was recently reported that topical butyrate improves the efficacy of 5-ASA in refractory distal ulcerative colitis[17], due to the presence of NaB in the colon administered topically in situ. Vernia et al[16] demonstrated in a pilot study that oral butyrate may improve the efficacy of oral mesalazine in active ulcerative colitis but a large scale investigation to confirm the present findings is still required. In this case the NaB was administered as tablets coated with a pH-dependent soluble polymer. More recently it has been reported that chronic feeding (tablets, 4 g a day for 8 wk) of this enteric coated NaB formulation (tablets, 4 g a day for 8 wk) (tablets, 4 g a day for 8 wk) can effectively induce clinical improvement/remission in mild Crohn’s disease[25].
An adequate enteric coating is needed for therapy of ileo-colonic disorders since when uncoated uncoated oral formulation of NaB is administered in an uncoated oral formulation, the compound is promptly dissolved and rapidly metabolized before reaching the colon as shown by the time of the maximum 13CO2 excretion occurring within 30-45 min after the 13C butyrate oral administration. A specific enteric coated formulation has been therefore designed to deliver the drug in that portion of the intestine keeping into account the high variability of the intestinal transit time observed in IBD patients.
The amount of 13CO2 excreted in breath was similar among the Crohn’s disease patients, showing that the rate and efficiency of NaB metabolism are similar in the healthy subjects. The patients were characterized by a mild active disease accounting for the efficient metabolism of butyrate and the oral formulation was expected to deliver NaB into the colon in a similar extent as to enema administration.
Furthermore the new coated 13C-NaB oral formulation containing 13C-labeled butyrate can also be used to evaluate the rate of colocyte-metabolized NaB by performing 13CO2 breath test before and after chronic administration of NaB. The impairment of NaB utilization by colonocytes that has been observed in previous studies[2-4] can then readily be evaluated by measuring the reduction in cumulative 13CO2 excretion in the 8-h period following oral administration of colon-targeted NaB tablets. A similar approach could also be used to evaluate the effectiveness of therapies for IBD patients with mesalazine (either alone or in combination with NaB) and new formulations designed to improve NaB absorption and metabolism. The reported NaB formulation can effectively improve oral administration of NaB ensuring the release of the active compound in the lower intestine portion which is the target for its pharmacological activity.
S- Editor Liu Y L- Editor Wang XL E- Editor Lu W
| 1. | Cummings JH. Short chain fatty acids in the human colon. Gut. 1981;22:763-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 713] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 2. | Roediger WE, Nance S. Metabolic induction of experimental ulcerative colitis by inhibition of fatty acid oxidation. Br J Exp Pathol. 1986;67:773-782. [PubMed] |
| 3. | Chapman MA, Grahn MF, Boyle MA, Hutton M, Rogers J, Williams NS. Butyrate oxidation is impaired in the colonic mucosa of sufferers of quiescent ulcerative colitis. Gut. 1994;35:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 169] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Chapman MA, Grahn MF, Hutton M, Williams NS. Butyrate metabolism in the terminal ileal mucosa of patients with ulcerative colitis. Br J Surg. 1995;82:36-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Finnie IA, Taylor BA, Rhodes JM. Ileal and colonic epithelial metabolism in quiescent ulcerative colitis: increased glutamine metabolism in distal colon but no defect in butyrate metabolism. Gut. 1993;34:1552-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Scheppach W, Sommer H, Kirchner T, Paganelli GM, Bartram P, Christl S, Richter F, Dusel G, Kasper H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology. 1992;103:51-56. [PubMed] |
| 7. | Steinhart AH, Hiruki T, Brzezinski A, Baker JP. Treatment of left-sided ulcerative colitis with butyrate enemas: a controlled trial. Aliment Pharmacol Ther. 1996;10:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 158] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Patz J, Jacobsohn WZ, Gottschalk-Sabag S, Zeides S, Braverman DZ. Treatment of refractory distal ulcerative colitis with short chain fatty acid enemas. Am J Gastroenterol. 1996;91:731-734. [PubMed] |
| 9. | Scheppach W. Treatment of distal ulcerative colitis with short-chain fatty acid enemas. A placebo-controlled trial. German-Austrian SCFA Study Group. Dig Dis Sci. 1996;41:2254-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 134] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Den Hond E, Hiele M, Evenepoel P, Peeters M, Ghoos Y, Rutgeerts P. In vivo butyrate metabolism and colonic permeability in extensive ulcerative colitis. Gastroenterology. 1998;115:584-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Simpson EJ, Chapman MA, Dawson J, Berry D, Macdonald IA, Cole A. In vivo measurement of colonic butyrate metabolism in patients with quiescent ulcerative colitis. Gut. 2000;46:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Säemann MD, Böhmig GA, Osterreicher CH, Burtscher H, Parolini O, Diakos C, Stöckl J, Hörl WH, Zlabinger GJ. Anti-inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000;14:2380-2382. [PubMed] |
| 13. | Coradini D, Pellizzaro C, Marimpietri D, Abolafio G, Daidone MG. Sodium butyrate modulates cell cycle-related proteins in HT29 human colonic adenocarcinoma cells. Cell Prolif. 2000;33:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Otaka M, Singhal A, Hakomori S. Antibody-mediated targeting of differentiation inducers to tumor cells: inhibition of colonic cancer cell growth in vitro and in vivo. A preliminary note. Biochem Biophys Res Commun. 1989;158:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Avivi-Green C, Polak-Charcon S, Madar Z, Schwartz B. Apoptosis cascade proteins are regulated in vivo by high intracolonic butyrate concentration: correlation with colon cancer inhibition. Oncol Res. 2000;12:83-95. [PubMed] |
| 16. | Vernia P, Monteleone G, Grandinetti G, Villotti G, Di Giulio E, Frieri G, Marcheggiano A, Pallone F, Caprilli R, Torsoli A. Combined oral sodium butyrate and mesalazine treatment compared to oral mesalazine alone in ulcerative colitis: randomized, double-blind, placebo-controlled pilot study. Dig Dis Sci. 2000;45:976-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Vernia P, Annese V, Bresci G, d'Albasio G, D'Incà R, Giaccari S, Ingrosso M, Mansi C, Riegler G, Valpiani D. Topical butyrate improves efficacy of 5-ASA in refractory distal ulcerative colitis: results of a multicentre trial. Eur J Clin Invest. 2003;33:244-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 154] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Pinto A, Fidalgo P, Cravo M, Midões J, Chaves P, Rosa J, dos Anjos Brito M, Leitão CN. Short chain fatty acids are effective in short-term treatment of chronic radiation proctitis: randomized, double-blind, controlled trial. Dis Colon Rectum. 1999;42:788-795; discussion 795-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Høverstad T, Bøhmer T, Fausa O. Absorption of short-chain fatty acids from the human colon measured by the 14CO2 breath test. Scand J Gastroenterol. 1982;17:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Tuleu C, Andrieux C, Cherbuy C, Darcy-Vrillon B, Duée PH, Chaumeil JC. Colonic delivery of sodium butyrate via oral route: acrylic coating design of pellets and in vivo evaluation in rats. Methods Find Exp Clin Pharmacol. 2001;23:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Azzaroli F, Mazzella G, Mazzeo C, Simoni P, Festi D, Colecchia A, Montagnani M, Martino C, Villanova N, Roda A. Sluggish small bowel motility is involved in determining increased biliary deoxycholic acid in cholesterol gallstone patients. Am J Gastroenterol. 1999;94:2453-2459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439-444. [PubMed] |
| 23. | Wilson FA. Intestinal transport of bile acids. Handbook of physiology. The gastrointestinal system. Intestinal absorption and secretion. Bethesda: Am Physiol Soc 1991; 389-404. |
| 24. | Aldini R, Montagnani M, Roda A, Hrelia S, Biagi PL, Roda E. Intestinal absorption of bile acids in the rabbit: different transport rates in jejunum and ileum. Gastroenterology. 1996;110:459-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Di Sabatino A, Morera R, Ciccocioppo R, Cazzola P, Gotti S, Tinozzi FP, Tinozzi S, Corazza GR. Oral butyrate for mildly to moderately active Crohn's disease. Aliment Pharmacol Ther. 2005;22:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |