Published online Feb 21, 2007. doi: 10.3748/wjg.v13.i7.1042
Revised: December 27, 2006
Accepted: January 4, 2007
Published online: February 21, 2007
AIM: To elucidate the sequential transfer of iron amongst ferritin, transferrin and transferrin receptor under various iron status conditions.
METHODS: Incorporation of 59Fe into mucosal and luminal proteins was carried out in control WKY rats. The sequential transfer of iron amongst ferritin, transferrin and transferrin receptor was carried out in iron deficient, control and iron overloaded rats. The duodenal proteins were subjected to immunoprecipitation and quantitation by specific ELISA and in situ localization by microautoradiography and immunohistochemistry in tandem duodenal sections. Human duodenal biopsy (n = 36) collected from subjects with differing iron status were also stained for these proteins.
RESULTS: Ferritin was identified as the major protein that incorporated iron in a time-dependent manner in the duodenal mucosa. The concentration of mucosal ferritin was significantly higher in the iron excess group compared to control, iron deficient groups (731.5 ± 191.96 vs 308.3 ± 123.36, 731.5 ± 191.96 vs 256.0 ± 1.19, P < 0.005), while that of luminal transferrin which was significantly higher than the mucosal did not differ among the groups (10.9 ± 7.6 vs 0.87 ± 0.79, 11.1 ± 10.3 vs 0.80 ± 1.20, 6.8 ± 4.7 vs 0.61 ± 0.63, P < 0.001). In situ grading of proteins and iron, and their superimposition, suggested the occurrence of a sequential transfer of iron. This was demonstrated to occur through the initial binding of iron to luminal transferrin then to absorptive cell surface transferrin receptors. The staining intensity of these proteins varied according to the iron nutrition in humans, with intense staining of transferrin receptor observed in iron deficient subjects.
CONCLUSION: It is concluded that the intestine takes up iron through a sequential transfer involving interaction of luminal transferrin, transferrin-transferrin receptor and ferritin.
- Citation: Kolachala VL, Sesikeran B, Nair KM. Evidence for a sequential transfer of iron amongst ferritin, transferrin and transferrin receptor during duodenal absorption of iron in rat and human. World J Gastroenterol 2007; 13(7): 1042-1052
- URL: https://www.wjgnet.com/1007-9327/full/v13/i7/1042.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i7.1042
Iron homeostasis is accomplished by regulating absorption in the proximal small intestine and is regulated according to the body’s needs. Failure to maintain this equilibrium leads to pathological conditions resulting in either iron deficiency or iron overload. Iron deficiency anemia remains the most important micronutrient deficiency world wide. Iron is essential because of its unique ability to serve as both an electron donor and acceptor. Because of iron’s virtual insolubility and potential toxicity under physiological conditions, special molecules have evolved for its acquisition, transport and storage in soluble, nontoxic form. In humans, heme iron is absorbed more efficiently than non-heme iron[1]. Recent studies demonstrate that non-heme iron is transported into the cell in the ferrous [Fe (II)] form, mainly by carrier divalent metal transporter 1 (DMT1)-also known as natural resistance associated macrophage protein 2 (Nramp2) or divalent cation transporter 1 (DCT1)[2] . However, iron absorption is not impaired by mutation of DMT-1, suggesting that DMT-1 is not the only transporter operating within the endosomes of crypt cells. Studies of Conrad et al[3], showed that ferric iron is absorbed by β3 integrin and mobilferrin pathway which is shared with other nutritional metals .
In last few decades, several candidate proteins involved in the transmembrane transport of iron have been identified[4] . Several years ago, Granick et al demonstrated that ferritin sequesters iron and provides the block to iron uptake. Although this model remains unproven, the hypothesis that the amount of mucosal ferritin dictates the extent of iron absorbed by the enterocytes is still intriguing. It has been postulated that iron absorption was the primary method of maintaining body iron homeostasis and was regulated by a mucosal receptor that blocked iron absorption when it became satiated with iron. For two decades, it was believed that ferritin was the receptor that regulates iron absorption, and that apoferritin enhances iron absorption while holoferritin blocks iron uptake. This hypothesis was disproved by immunological studies showing that there was little or no apoferritin in the absorptive cells of iron deficient animals[5]. Despite the close relationship between ferritin levels and intestinal iron absorption[6] it is not clear whether this protein plays a passive or active role in transport regulation.
There are studies to argue for[7-11] and against[12-14] the view that transferrin (Tf) is an important mediator of iron absorption. The proposal that mucosal Tf acts as a shuttle protein for iron absorption was suggested by Huebers et al[15]. Selective localization of transferrin in the duodenal epithelium in rat[16,17] and in human[17] , and the co-localization of transferrin receptor (TfR) and transferrin in coated pits on the basal and lateral membranes of crypt cells in mouse have been demonstrated[18]. However, an ultrastructural study of Parmley et al[19] failed to identify TfR on the surface of enterocyte microvilli. Thus, the role of transferrin and transferrin receptor in the duodenal uptake was not identified with certainty. In 1983, it was postulated that transferrin was secreted into the intestinal lumen to bind iron and enter the absorptive cell as a transferrin iron complex in a manner similar to non-intestinal cells[15]. Recent studies of transferrin receptor knockout mice (TfR-/-) provide new insights regarding the physiologic role of the transferrin/transferrin receptor cycle. Homozygous TfR-/- animals die in utero with impaired erythropoiesis and defective neurological development. Thus, the TfR-/- mice provide a convincing demonstration that the transferrin/transferrin receptor cycle plays a central role in the maintenance of normal iron metabolism. Whether transferrin is associated with receptor and whether it plays any role in iron absorption at this site is yet to be determined. A protein called hereditary hemochromatosis (HFE) has been shown to interact with transferrin receptor 1 (TfR1)[20] to influence the rate of receptor mediated uptake of transferrin-bound iron[21-24] . Thus, these investigations of exact localization of mucosal transferrin and transferrin receptor, inside or outside the enterocytes, are conflicting. Therefore, an attempt was made to describe the sequential movement of iron across intestine. The events were sequenced by in situ localization of iron by microautoradiography and immunohistochemistry of ferritin, transferrin and transferrin receptor in serial sections of intestine.
Radioactive 59FeCl3 (sp.act 4.0 Ci/g iron in 0.01 mol/L HCl) was obtained from BRIT (Mumbai, India). Photographic emulsion LM-1 was obtained from Amersham (Amersham International plc, UK). SIH universal anti-rabbit kit, human and rat serotransferrin was obtained from Sigma Aldrich Co (St. Louis, USA). Ferritin, transferrin antisera against purified proteins were produced in New Zealand white rabbits. Polyclonal antiserum against rat placental transferrin receptor was a gift from James D. Cook, M.D (University of Kansas Medical Center, Kansas City, USA). All other chemicals used were of analytic grade and procured locally.
Rats from National Centre for Laboratory Animal Sciences (National Institute of Nutrition, Hyderabad) were used for the study. All animal experiments were approved by the institutional animal ethics committee.
Incorporation of radioactive iron into various iron binding protein was carried out in Wistar/Kyoto (WKY) adult male normal rats (body weight 200 g). Food was withheld for 16-18 h before administering 1 mL of 7-10 μci of 59FeCl3 in 0.01N HCl by gavage. Rats were sacrificed at various time intervals starting at 15, 30 min, 1, 2, 4 and 12 h to study the time-dependent incorporation of iron into luminal and mucosal proteins of various segments. All the subsequent operations were carried out on ice. The luminal content was collected by flushing with 5-10 mL of saline containing protease inhibitors. The mucosal scrapings were obtained by scrapping off the everted segments with a glass slide. The intestinal contents obtained from three rats from each group were pooled together for each time point. The contents were homogenized in 1:4 v/v of saline containing a cocktail of protease inhibitors (PMSF 75 μg/mL, leupeptin 1 μg/mL and iodoacetate186 μg/mL), and subjected to 60% ammonium sulphate fractionation. A clear supernatant was prepared for subsequent analysis.
Further to explore the specific role of duodenal transferrin and ferritin during iron absorption, time-dependent (5 min-4 h) incorporation of radioactive iron into these proteins, along with quantitation by specific ELISA and immunoprecipitation techniques for ferritin and transferrin, was carried out in control, iron-deficient, and excess iron fed rats. For this, 74 WKY male weanling rats (body weight 37.5 ± 7.63) were randomly selected and housed individually into iron deficient (n = 30), control (n = 24) and iron excess (n = 24) groups. They were allowed to have free access to food and water for 7 weeks. The rats were placed on one of the three diets each containing identical protein, fat, carbohydrate, and complete vitamin and fiber supplements. The diets were produced in accordance with the recommendations of American Institute of Nutrition (AIN93)[25]. The diet also contained a balanced mineral mix, differing only in the iron content. The deficient group received iron deficient semi-synthetic diet containing < 10 mg iron/kg diet, while the iron adequate and excess groups received diet containing 35 and 250 mg/kg diet, respectively. At the end of this period rats were administered 7-10 μCi of radioactive iron and sacrificed at different time points. Duodenal segments of intestine were collected at 5, 15, 30 min, 1, 2, 4 h and processed. Homogenates of luminal and mucosal contents were analyzed for ferritin and transferrin by specific sandwich ELISA. Incorporation of radiolabelled iron into these proteins was studied by imunoprecipitation with specific antibodies.
Finally, studies were carried out to understand the sequential movement of iron across intestine under various iron status conditions. WKY male weanling rats (body weight 36.8 ± 6.56) were equally (n = 8) distributed into iron deficient, control and iron overload groups. For iron overloading, in addition to iron excess diet as mentioned above, we gave 4 intra-peritonial injections of 1 mL of imferon, an iron sorbitol citric acid complex in water (50 mg of iron/mL, Rallies India Ltd, Mumbai, India) at weekly intervals. After 7 wk, rats were fasted overnight and given 100 μCi of 59FeCl3 with 0.25 mg of carrier iron as ferrous sulphate by gavage. Duodenal segment of intestine were collected at 5, 15, 30 min, 1, 2, 4, 16 h. The segments were flushed with formalin, cut opened longitudinally and fixed in 10% neutral formalin for 12 h. These intestinal segments were rolled longitudinally (Swiss-roll), and further processed for microautoradiography and immunohistochemistry.
The protein content was estimated by the method of Bradford[26]. Both luminal and mucosal proteins (100 μg) were subjected to 4%-20% PAGE along with rat liver ferritin as a marker. The separated protein bands were visualized by autoradiography and probed with ferritin antibody.
Ferritin and transferrin proteins in the luminal and mucosal contents were quantified using a specific sandwich ELISA system developed by us.
In order to understand the role of different iron binding proteins during absorption, luminal contents and mucosal proteins were lyophilized. Duodenal mucosal and luminal proteins obtained at initial time points (5, 15, 30 min) were reconstituted and subjected to immunoprecipitation with ferritin and transferrin antisera. Equal volumes (1 mL) of duodenal mucosal and luminal proteins (2-4 mg) and 1:32 diluted ferritin and transferrin antisera were incubated at 37°C for 1 h and at 4°C overnight. The specificity of ferritin and transferrin antisera was demonstrated by replacing immune serum with non-immune serum in the immunoprecipitation protocol. The immunoprecipitate was collected and washed three times with PBS. 59Fe radioactivity in the fraction was counted in a gamma counter (Packard Autogamma, Cobra II). A known activity of 59FeCl3 was run with samples to correct for decay and counting efficiency of the gamma counter.
An automatic tissue processor (Shandon, Processor 2LE) using ascending grades of isopropyl alcohol and chloroform was used. The processed tissue samples were embedded in paraffin (58-60°C) using a Leica tissue-embedding unit. A set of 10 serial sections of 4 μ thickness were taken from each block using Reichert-Jung 2030 rotary manual microtome. The sections were mounted on chromalum-gelatin coated glass slides and further processed for immunohistochemistry and microautoradiography according to standard procedures.
In situ localization of radiolabelled iron was carried out in dehydrated sections. The sections were processed with photographic emulsion according to the manufacturer’s guidelines (Amersham LM-1, Amersham UK). These sections were stained with hematoxylin, dehydrated and mounted with DPX mounting medium. For signals, the sections were viewed under light microscope (Leitz Ortholux) and photographed.
Serial sections were used for immunohistochemical localization of transferrin, transferrin receptor and ferritin, using respective antisera. The binding of each antiserum to their respective proteins was done using Sigma SIH kit. Counter-staining was done with Mayer’s hematoxylin. Control slides were layered with non-immune serum instead of primary antiserum. The localization of the antigen was done using goat anti-rabbit HRPO conjugate. The comparison of staining intensity and the quantification of positively stained cells was carried out under light microscope with the magnification set at 10 ×, 25 × or 40 ×. In addition, the distribution of intestinal mucosal ferritin, transferrin and transferrin receptor in relation to iron status was evaluated in human biopsy specimens.
Endoscopic intestinal (duodenal) biopsy specimens were collected by a gastroenterologist from 30 males and 6 females attending the Gastroenterology Department of Gandhi Hospital (Secunderabad, India) for various upper GI tract related ailments. Informed oral consent was obtained from all the subjects. All the subjects were classified based on their hematological and iron status parameters. Accordingly, a cut off value of hemoglobin < 13 g/dL for male and < 12 g/dL for female was classified as anemia, while anemia with serum ferritin < 12 μg/L as iron deficiency anemia. Biopsy specimens of 3-4 mm were collected and immediately spread on a wire mesh. These specimens were then immersed in 10% neutral buffered formalin solution and processed for immunohistochemistry of ferritin, transferrin and transferrin receptor, as described earlier.
Fasting blood samples were collected from rats and human subjects to estimate haemoglobin and iron status parameters. Hemoglobin was estimated by an automated hematology counter (Serono system 9000 Rx). Quantitation of human and rat serum ferritin, rat mucosal, and luminal ferritin and transferrin was estimated by homologous sandwich ELISA systems developed by us. Liver iron was estimated by dry ashing followed by an estimation of iron in the mineral solution by bathophenathroline method[1].
Initially, a kinetic study was carried out to understand the in vivo time course of iron absorption of 59Fe given by oral gavage to rats with normal iron status condition. Specific activity of duodenal mucosa was found to be highest, followed by jejunum, ileum and stomach at all time points studied. The specific activity of 59Fe was found to increase with time and attained a peak at 30 min in duodenal mucosa (data not shown).
In order to understand the role of ferritin in iron absorption, an autoradiogram was performed with proteins obtained from 60% ammonium sulphate precipitation of luminal and mucosal fractions after oral administration of 59Fe. As shown in Figure 1, upper panel, rats with normal iron status showed a single radioactive protein band identical to purified rat liver ferritin. The intensity of the radioactive band associated with ferritin increased with time and showed maximal band intensity at 30 min and decreased subsequently with no change in the ferritin protein band intensity (Figure 1, lower panel). These results suggested that intestinal ferritin is an important component of the intestinal iron transport system and seems to take up iron and facilitate its transfer across the mucosal cells. Thus these results demonstrate that ferritin is not just a sink but takes up iron and releases it in a time-dependent manner during the absorptive process.
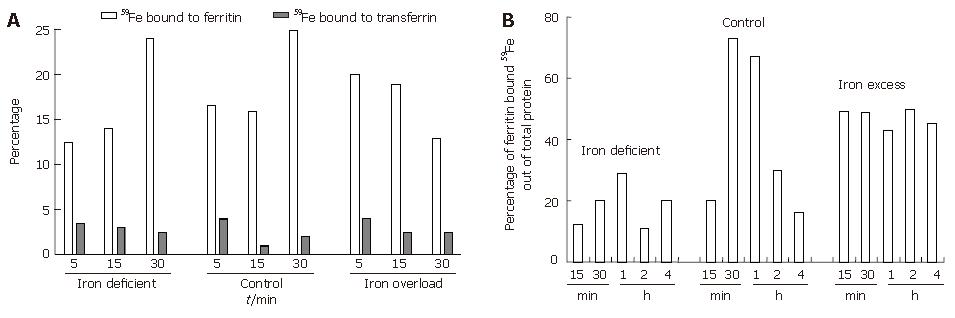
To determine the specific role of iron binding proteins like ferritin, transferrin and transferrin receptor, rats were given different amounts of iron. Haematological and iron status parameters indicated the induction of iron deficiency and iron overloading in respective groups (data not shown). Table 1 shows the ferritin and transferrin concentrations in the intestinal luminal and mucosal contents of rats with various iron status parameters. The concentration of ferritin was higher in mucosa than in the lumen of duodenum in all the groups. In iron excess group, there was a significantly higher concentration of ferritin, both in lumen and mucosa, compared to deficient and control groups. In contrast, the transferrin concentration was significantly higher in lumen than in mucosa within the group and was similar between the groups.
| Parameter | Control | Iron deficient | Iron excess |
| Ferritin (ng/mg protein) | |||
| Lumen | 126.0a± 61.42 | 105.8a± 35.81 | 228.8c± 73.54 |
| Mucosa | 308.3c± 123.36 | 256.0c± 1.19 | 731.5e± 191.96 |
| Transferrin (μg/mg protein) | |||
| Lumen | 10.9b± 7.6 | 11.1b± 10.3 | 6.8b± 4.7 |
| Mucosa | 0.87d± 0.79 | 0.80d± 1.20 | 0.61d± 0.63 |
To understand the interaction between luminal transferrin and mucosal ferritin after oral administration of 59Fe, rats were sacrificed at 5, 15 and 30 min. As shown in Figure 2A the percentage of radioactive iron in duodenal lumen was found to decrease with time. The highest luminal specific activity was seen in iron-deficient rats followed by control and iron excess fed rats. In deficient and control rats, there was a progressive increase in specific activity while in the excess iron fed group, the radioactivity was retained at the site of absorption (Figure 2B). This was supported by the findings on luminal and mucosal radioactivity, which showed a faster transit of iron from lumen to mucosa in iron-deficient and control rats. On the other hand, in the iron excess fed rats luminal radioactivity showed slower transit as indicated by increase in the radioactivity from 30 min onwards. The plasma radioactivity during the same time periods reflected the slower transit of duodenal iron in iron excess fed rats (plasma specific activity of 150 ± 20 dpm/mL during 15-60 min) which showed no peak in plasma iron as compared to the iron-deficient and control groups (plasma specific activity of 700 ± 50 and 400 ±50 dpm/mL, respectively). The luminal transferrin iron declined with time in all the groups while that of mucosal ferritin increased only in deficient and sufficient groups during the 5-30 min of iron uptake (Figure 2). As shown in Figure 2B percentage of ferritin bound iron reached a peak and showed a decline confirming the release of iron from ferritin in deficient and control groups. In the iron excess group the percentage ferritin-bound iron did not change with time, confirming the blockage of iron in this group.
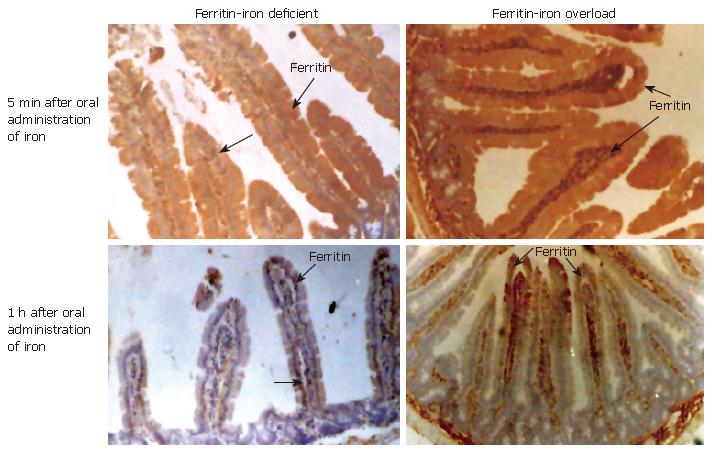
In order to study the in vivo translocation of iron after oral administration of radiolabelled iron, duodenal segments were processed for microautoradiography under various iron status conditions. Table 2 shows the grading of staining intensities of radiolabelled iron, ferritin, transferrin/transferrin receptor in the iron deficient and iron excess groups. The radioactivity at 5 min was localized on the surface of the villi in the iron deficient group (top left panel ID, 5 min) (Figure 3). Subsequently, the radioactivity was present in the columnar epithelial cells (absorptive cells) at the tip of the villi between 15 min to 1 h in iron deficient (top right, panel ID, 15, 30 min, 1 h, and Table 2). At 2 and 4 h most of the radioactive iron was seen inside the villi in the lamina propria. A similar trend was observed in the control group (middle, panel C, 5 min and 15, 30 min, 1 h). However, in iron-overloaded intestine, high intensity radioactivity was noticed at 5 min on the surface (bottom left, panel OL, 5 min) but was minimal signals at 15 and 30 min (bottom right panel OL, 15 min and Table 2). The radioactive signals at 15 and 30 min associated with iron were maximal intraepithelially in iron-deficient rats and plasma radioactivity indicated maximum absorption. In iron-overloaded rats the signal was maximal at 5 min and seen only at the absorptive surface and retained at the site of absorption (Table 2).
| Radioactiveiron/protein | Iron status | Basal | 5 min | 15 min | 30 min | 2h |
| 59Fe | ID | - | SUR+ | IE+++ | IE+++ | - |
| OL | - | SUR+++ | IE± | IE± | - | |
| Tf | ID | IV± | IV++ | IE+++ | IE+ | |
| IV++ | IV++ | |||||
| OL | IV± | IE± | IE± | |||
| IV± | IV± | |||||
| TfR | ID | SUR± | SUR++ | IE++ | IE+ | SUR± |
| OL | SUR± | SUR++ | SUR+ | - | - | |
| Fe | ID | IE± | IE± | |||
| IV± | - | - | ||||
| OL | IE± | - | IE± | IE± | ||
| IV+++ |
Immunohistochemistry with ferritin antibody demonstrated that at 5 min, ferritin staining was maximal intraepithelially in iron-deficient intestine (Figure 4), while intense staining was seen both intraepithelially and intravascularly, in iron-overloaded intestine (Figure 4 top right, panel OL, 5 min Fe). The intensity of staining within the villi at 1 h (bottom left panel ID-1 h, Fe) was minimal and similar to that seen at basal status in iron deficient intestine. On the other hand, the staining intensity was lower in iron-loaded intestine after 15 min indicating the presence of holoferritin and blocking of iron absorption (Table 2).
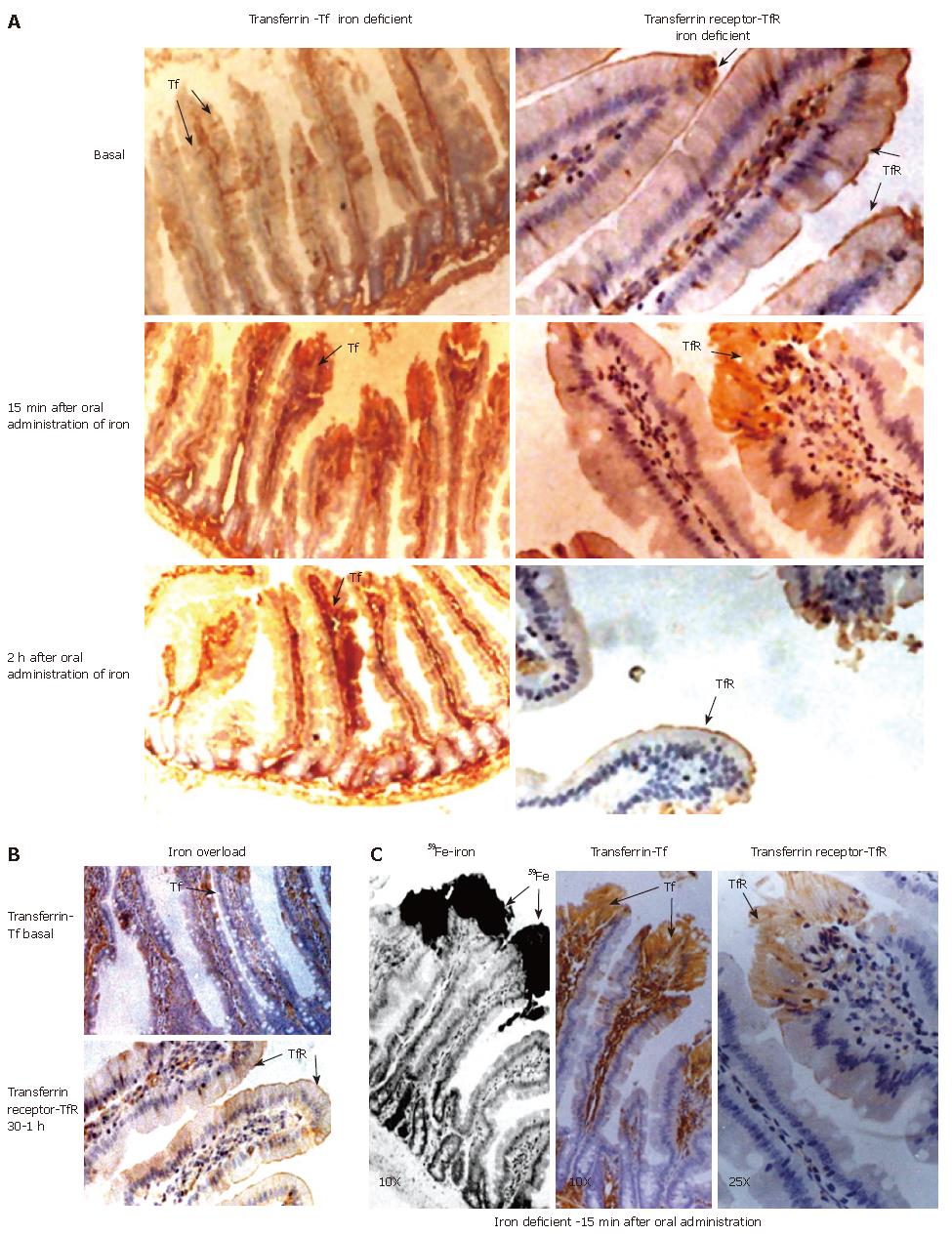
The in situ localization of radioactive iron in the iron deficient group indicated its presence on the cell surface (5 min) and then intraepithelially (15, 30 min) in the villi (Table 2). The intensity of transferrin staining was found to be maximal at the tip of the epithelial cells at 15 min (Figure 5, left panel ID 15 min Tf) and subsequently decreased to basal status. Staining of the blood vessels for transferrin started to appear from 2 h onwards in ID (bottom left panel ID, 2 h Tf). The staining for transferrin at 15 min within the epithelial cells was not observed in iron-overload group (Table 2). In the iron-overload group, staining due to transferrin was minimal at 5-30 min (Figure 5B, top left panel Tf, OL 30 min). In both groups, the transferrin receptor was localized mainly on the surface of the duodenal villi, thus demonstrating margination of transferrin receptor after oral administration of iron (right top panel Figure 5A, TfR, Basal). This, however, at 15 min was internalized at the tip of the epithelial cells along with iron and transferrin (Figure 5A). After 2hr TfR reappeared on the surface of the villi in the iron deficient (Figure 5A, middle right panel, TfR, ID, 15 min, 2 h) demonstrating the in vivo receptor recycling. In contrast, in the iron-overload group TfR was seen only at the surface even at 30 min and 1 h (Figure 5B, bottom panel, TfR, OL, 15 min, 1h) showing no internalization of receptor.
The radioactive signals at 15 min were maximal intraepithelially in the iron deficient group. During this time period, transferrin and its receptor were also co-localized maximally within the epithelial cells (Figure 5C). The intensities of both these proteins subsequently diminished and minimal staining was seen at the margin of the absorptive surface. The intensity of transferrin receptor staining was maximal along the entire margin during different time points in the iron-overloaded intestine. This was a striking difference between the two extreme conditions. Microautoradiography along with the immunohistochemistry of ferritin and the transferrin/transferrin receptor demonstrated the in vivo internalization of transferrin and its receptor along with radiolabelled iron and recycling of the receptor to the surface. This clearly shows a transferrin-mediated iron uptake at the absorptive surface of the rat intestine.
In addition, we have performed immonohistochemistry of ferritin, transferrin/transferrin receptor in human duodenal biopsy specimens collected from various iron status conditions. All the subjects were classified according to their indicators of iron status such as haemoglobin, serum ferritin (Table 3). According to these parameters, 13 of them belonged to normal group, 6 had iron-deficient anemia, 14 anemia, one each had blood transfused, iron injected and iron given orally as supplement. Representative immunohistochemical images for ferritin, transferrin and transferrin receptor of duodenal biopsy from normal, iron deficient anemia and blood transfused are given in Figure 6.
| Sex | No | Age (yr) | Hb (g/dL) | PCV | RBC M/cu mm | Serum ferritin (μg/L) | |
| Normal | M | 12 | 36.5 ± 10.6 | 14.8 ± 0.9 | 44.5 ± 3.3 | 5.8 ± 0.7 | 43 ± 22.1 |
| F | 1 | 7.2 | 12.4 | 36 | 4.5 | 71.7 | |
| Iron deficient anemia | M | 4 | 24.5 ± 20.9 | 7.4 ± 2.6 | 23.3 ± 9.0 | 4.1 ± 1.7 | 7.2 ± 4.0 |
| F | 2 | 20.5 | 7.3 | 26.5 | 3.5 | 6.5 | |
| M | 10 | 44.3 ± 16.5 | 11.1 ± 1.64 | 33.6 ± 5.2 | 4.5 ± 1.4 | 75.3 ± 32.2 | |
| Anemia | F | 4 | 21 ± 6.6 | 6.8 ± 3.0 | 20.8 ± 9.0 | 2.1 ± 1.2 | 57.4 ± 13.4 |
| Iron supplements | F | ||||||
| Blood transfusion | M | 1 | 32 | 9.5 | 29 | 4.7 | 28.6 |
| Iron injection | F | 1 | 32 | 8.4 | 27 | 4.3 | 101 |
| Oral iron | F | 1 | 20 | 9.7 | 30 | 3 | 38 |
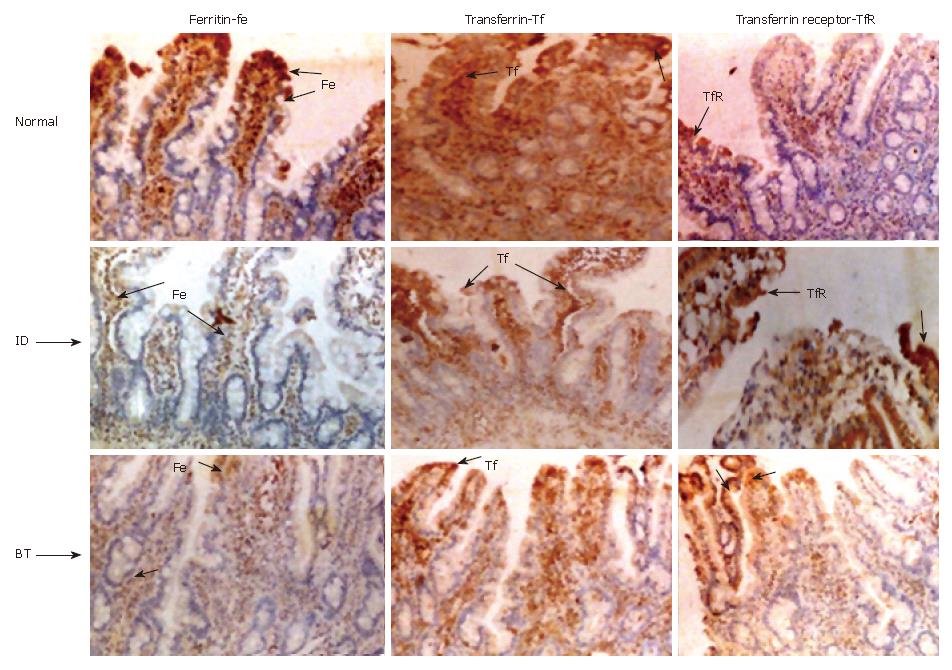
The staining intensity for ferritin varied according to iron status and thus staining was +++ normal (top left), ± iron deficient (middle left panel Fe ID) and ++ in blood transfused (Figure 6 bottom left panel Fe BT) subjects. The intensity of transferrin staining was also similar to that of ferritin staining in normal, iron-deficient and in blood transfused subjects (center top, middle and bottom panels respectively). The transferrin receptor on the other hand, stained more intensely with a grading score of (+++) in the iron-deficient subjects (Figure 6 middle right panel ID TfR) compared to + in normal (top right panel, TfR) and blood transfused subjects (bottom right panel BT TfR).
Ferritin, transferrin and transferrin receptor are the important proteins that regulate iron homeostasis in almost all the cells. However, the actions of these proteins in the absorptive cells remain unclear. Data on the role of these proteins were obtained mainly by studying one protein at a time. By attempting to sequence serially the role of these proteins together, during intestinal uptake, we were able to provide evidence for in vivo receptor mediated uptake of iron.
The radioactive signals at 15 to 30 min associated with iron were maximal intraepithelially in iron deficient rats. Along with iron, both transferrin and transferrin receptor were co-localized at this time. The intensities of both these proteins were subsequently decreased and minimal amounts were seen at the margin of absorptive surface in iron deficient intestine. In addition, ferritin appeared intraepithelial in iron deficient and control groups, suggesting its modulation during the absorptive phase. Presence of most of the radiolabelled signal only on absorptive surface at 5 min and beyond in iron-overloaded rats suggest its restricted entry into the duodenum due to the presence of mucosal holo ferritin. This is supported by the intense ferritin staining seen both intraepithelial and intravascular in iron overloaded intestine, especially during absorptive phase and beyond. Further, the presence of transferrin receptor along the entire margin during different phases of iron uptake suggests no internalization of transferrin receptor in iron excess condition.
It is interesting to note that the concentration of mucosal ferritin and the iron associated with it was similar in control and iron deficient groups, and was significantly lower than that seen in the excess iron fed group. This could be due to the regulatory role of mucosal ferritin at the site of adsorption in the case of iron excess, while in the case of normal and iron deficient duodenum it could release the incorporated iron.
A positive relationship between in situ localization of ferritin and better iron status was demonstrated in rats and humans. The results on autoradiography of rat duodenal mucosal and luminal preparations suggests that intestinal ferritin takes up iron and releases it in a time dependent manner during the absorptive process. This is supported by the findings on localization of iron in the duodenal mucosa during absorption which showed a positive relationship, with better iron status at 5 min and a negative relationship at 15 min-1h. Similarly in human subjects, the intensity of ferritin staining was lowest in iron deficient subjects, highest in normal subjects and in between in the case of the blood transfused subjects. These observations suggest that mucosal ferritin is an important component of the intestinal iron transport system which takes up iron and facilitates its transfer across the mucosal cells.
The studies of Miyoshi et al[27] support the presence of ferritin in duodenum. Their studies showed accumulation of iron in the apical area of duodenal villous cells with in ferritin. The presence of maximal 55Fe radioactivity at 5 min over the brush border and terminal web was demonstrated by Bedard et al[28]. They observed that during the next 3 h, radioactivity was present almost exclusively in absorptive cells and in lamina propria. In contrast, Conrad and Crosby[29] observed very little radioactivity in the small intestinal mucosa of iron-depleted and iron overload rats from 2-48 h after gastric administration of 59Fe. This discrepancy may be due to the fact that they did not included time points earlier than 2 h. Because it is an inducible protein, it is possible that ferritin expression at the sites of absorption may be related to the iron status. This suggests that iron absorption is probably dependent on the relative concentrations of mucosal apo and holo ferritins, and is inhibited in conditions of iron overload and enhanced in iron deficiency.
Quantitatively, the amount of transferrin in the lumen is 10 times higher than that of mucosa and is not dependent on iron nutritional status (Table 1). The observation on identical amounts of luminal transferrin, irrespective of iron nutritional status, implies that this protein may be presenting equal amount of iron for absorption. The net amount of iron, which is taken up by the mucosa, thus depends on mucosal transferrin receptors. Duodenal mucosal transferrin receptor showed reciprocal relationship with iron status. The significance of this may be to present equal amounts of iron for absorption through binding to luminal transferrin. Consequently, the amount of iron absorbed by the mucosa may depend only on the mucosal transferrin receptor which has inverse relationship with better iron status.
Huebers et al[8,15] suggest that luminal transferrin acts as a shuttle protein for iron uptake. Autoradiographic studies by Conrad and Crosby[30] and Bedard et al[28] also suggest that intestinal crypt cells are able to take up transferrin-bound iron from circulation. According to some other investigators, however, transferrin is unlikely to be a major transporter of iron from lumen to the baso-lateral membrane[31-33]. According to Idzerda[34], the transferrin gene is not expressed in the intestine and, therefore, cannot be synthesized. Thus, it is possible that the luminal transferrin may be imported from plasma, as hypothesized by Parmley et al[19] .
Although the receptor mediated cellular uptake of iron is well known and takes place in all dividing cells, such a mechanism operating in the intestine has not been documented earlier. The results of this study, in conjunction with earlier observations[5,35-37], allow us to propose a model for intestinal iron absorption whereby., the recycling of transferrin and transferrin receptors enables luminal transferrin bound iron to enter the enterocytes. The receptor density at the absorptive surface, which is inversely proportional to better iron nutritional status, might regulate this iron uptake process.
The presence of transferrin and its receptor on the margin during basal condition, and the appearance of both the receptor and the ligand at 15 min intraepithelially along with the radiolabelled iron in iron depleted rat intestine, support the above model. The appearance of TfR after 2 h on the margin of villi suggests that the receptor protein returned to the surface after releasing iron. In contrast, there was no evidence of intraepithelial transferrin and transferrin receptor in iron overloaded intestine, as evidenced by the presence of receptor on the margin even at 30 min.
Though the staining intensity of transferrin in duodenal section was high in control subjects, there was no obvious difference between iron deficient subject and blood transfused subjects. Staining for transferrin was intense in the apical portion and blood vessels, while that of transferrin receptor was more concentrated at the epithelial lining of duodenum (Figure 6). The higher intensity of transferrin receptor in iron deficient subjects and minimal in control and blood transfused subjects suggests a role for it in iron absorption. This is supported by the reciprocal relationship observed between transferrin receptor and ferritin staining. Similar finding on TfR staining at the margin of the villi was observed by Banerjee et al[36] . The ultrastructural studies of Levine et al[31] have shown that transferrin receptor and transferrin are co-localized in coated pits on the basal and lateral membranes of crypt cells. Further, Anderson et al[35] confirmed that the TfR is a prominent protein on the basal and lateral membranes of intestinal epithelial cells.
The above findings, along with earlier observation, suggest that luminal transferrin must be presenting equal amount of iron for absorption since this protein does not respond to iron status. The net amount of iron taken up by mucosa depends on mucosal transferrin receptors, which respond reciprocally with better iron status. These results suggest that mucosal ferritin, luminal transferrin and mucosal cell surface transferrin receptors are closely related to iron status and interact with each other in carrying iron across the intestinal mucosa.
Vasantha L Kolachala was the recipient of an Indian Council of Medical Research Fellowship. Grateful acknowledgement also to Dr. Rupender Prasad (Mahavir Hospital, Hyderbad, India), and Dr. A Vidyasagar and Dr. B Prahlad (Gandhi Hospital, Hyderabad, India) for their help in providing human biopsy specimens.
S- Editor Liu Y L- Editor Banati RB E- Editor Ma WH
| 1. | Bothwell TH, Charlton RW. Current problems of iron overload. Recent Results Cancer Res. 1979;69:87-95. [PubMed] |
| 2. | Touret N, Furuya W, Forbes J, Gros P, Grinstein S. Dynamic traffic through the recycling compartment couples the metal transporter Nramp2 (DMT1) with the transferrin receptor. J Biol Chem. 2003;278:25548-25557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Conrad ME, Umbreit JN, Moore EG, Hainsworth LN, Porubcin M, Simovich MJ, Nakada MT, Dolan K, Garrick MD. Separate pathways for cellular uptake of ferric and ferrous iron. Am J Physiol Gastrointest Liver Physiol. 2000;279:G767-G774. [PubMed] |
| 4. | Conrad ME, Umbreit JN. Pathways of iron absorption. Blood Cells Mol Dis. 2002;29:336-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Brittin GM, Raval D. Duodenal ferritin synthesis during iron absorption in the iron-deficient rat. J Lab Clin Med. 1970;75:811-817. [PubMed] |
| 6. | Whittaker P, Skikne BS, Covell AM, Flowers C, Cooke A, Lynch SR, Cook JD. Duodenal iron proteins in idiopathic hemochromatosis. J Clin Invest. 1989;83:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Pollack S, Lasky FD. A new iron-binding protein isolated from intestinal mucosa. J Lab Clin Med. 1976;87:670-679. [PubMed] |
| 8. | Huebers H, Huebers E, Rummel W, Crichton RR. Isolation and characterization of iron-binding proteins from rat intestinal mucosa. Eur J Biochem. 1976;66:447-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Osterloh K, Forth W. Determination of transferrin-like immunoreactivity in the mucosal homogenate of the duodenum, jejunum, and ileum of normal and iron deficient rats. Blut. 1981;43:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Johnson G, Jacobs P, Purves LR. Iron binding proteins of iron-absorbing rat intestinal mucosa. J Clin Invest. 1983;71:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Cox TM, Mazurier J, Spik G, Montreuil J, Peters TJ. Iron binding proteins and influx of iron across the duodenal brush border. Evidence for specific lactotransferrin receptors in the human intestine. Biochim Biophys Acta. 1979;588:120-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 103] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Simpson RJ, Osterloh KR, Raja KB, Snape SD, Peters TJ. Studies on the role of transferrin and endocytosis in the uptake of Fe3+ from Fe-nitrilotriacetate by mouse duodenum. Biochim Biophys Acta. 1986;884:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Worwood M, Edwards A, Jacobs A. Non-ferritin iron compound in rat small intestinal mucosa during iron absorption. Nature. 1971;229:409-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Huebers HA, Huebers E, Csiba E, Rummel W, Finch CA. The significance of transferrin for intestinal iron absorption. Blood. 1983;61:283-290. [PubMed] |
| 16. | Isobe K, Isobe Y. Localization of transferrin in rat duodenal mucosa by immunoperoxidase technique. Nihon Ketsueki Gakkai Zasshi. 1983;46:797-807. [PubMed] |
| 17. | Mason DY, Taylor CR. Distribution of transferrin, ferritin, and lactoferrin in human tissues. J Clin Pathol. 1978;31:316-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 148] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Levine DS, Woods JW. Immunolocalization of transferrin and transferrin receptor in mouse small intestinal absorptive cells. J Histochem Cytochem. 1990;38:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Parmley RT, Barton JC, Conrad ME. Ultrastructural localization of transferrin, transferrin receptor, and iron-binding sites on human placental and duodenal microvilli. Br J Haematol. 1985;60:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Parkkila S, Waheed A, Britton RS, Bacon BR, Zhou XY, Tomatsu S, Fleming RE, Sly WS. Association of the transferrin receptor in human placenta with HFE, the protein defective in hereditary hemochromatosis. Proc Natl Acad Sci USA. 1997;94:13198-13202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 239] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Gross CN, Irrinki A, Feder JN, Enns CA. Co-trafficking of HFE, a nonclassical major histocompatibility complex class I protein, with the transferrin receptor implies a role in intracellular iron regulation. J Biol Chem. 1998;273:22068-22074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 170] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Roy CN, Blemings KP, Deck KM, Davies PS, Anderson EL, Eisenstein RS, Enns CA. Increased IRP1 and IRP2 RNA binding activity accompanies a reduction of the labile iron pool in HFE-expressing cells. J Cell Physiol. 2002;190:218-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Salter-Cid L, Brunmark A, Li Y, Leturcq D, Peterson PA, Jackson MR, Yang Y. Transferrin receptor is negatively modulated by the hemochromatosis protein HFE: implications for cellular iron homeostasis. Proc Natl Acad Sci USA. 1999;96:5434-5439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Waheed A, Grubb JH, Zhou XY, Tomatsu S, Fleming RE, Costaldi ME, Britton RS, Bacon BR, Sly WS. Regulation of transferrin-mediated iron uptake by HFE, the protein defective in hereditary hemochromatosis. Proc Natl Acad Sci USA. 2002;99:3117-3122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939-1951. [PubMed] |
| 26. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189576] [Cited by in RCA: 157385] [Article Influence: 3211.9] [Reference Citation Analysis (0)] |
| 27. | Miyoshi H, Ashida K, Hirata I, Ohshiba S, Naitoh T. Transferrin is not involved in initial uptake process of iron in rat duodenal mucosa. Ultrastructural study by x-ray energy spectrometry. Dig Dis Sci. 1995;40:1484-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 28. | Bédard YC, Pinkerton PH, Simon GT. [Iron absorption by the duodenal mucosa (author's transl)]. Nouv Rev Fr Hematol. 1973;13:727-743. [PubMed] |
| 29. | Crosby WH, Conrad ME, Wheby MS. The rate of iron accumulation in iron storage disease. Blood. 1963;22:429-440. [PubMed] |
| 30. | Bédard YC, Pinkerton PH, Simon GT. Uptake of circulating iron by the duodenum of normal mice and mice with altered iron stores, including sex-linked anemia: high resolution radioautographic study. Lab Invest. 1976;34:611-615. [PubMed] |
| 31. | Levine PH, Levine AJ, Weintraub LR. The role of transferrin in the control of iron absorption: studies on a cellular level. J Lab Clin Med. 1972;80:333-341. [PubMed] |
| 32. | Schümann K, Osterloh K, Forth W. Independence of in vitro iron absorption from mucosal transferrin content in rat jejunal and ileal segments. Blut. 1986;53:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Mazurier J, Montreuil J, Spik G. Visualization of lactotransferrin brush-border receptors by ligand-blotting. Biochim Biophys Acta. 1985;821:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Idzerda RL, Huebers H, Finch CA, McKnight GS. Rat transferrin gene expression: tissue-specific regulation by iron deficiency. Proc Natl Acad Sci USA. 1986;83:3723-3727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 119] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 35. | Anderson GJ, Powell LW, Halliday JW. The endocytosis of transferrin by rat intestinal epithelial cells. Gastroenterology. 1994;106:414-422. [PubMed] |
| 36. | Banerjee D, Flanagan PR, Cluett J, Valberg LS. Transferrin receptors in the human gastrointestinal tract. Relationship to body iron stores. Gastroenterology. 1986;91:861-869. [PubMed] |
| 37. | El-Shobaki FA, Rummel W. Mucosal transferrin and ferritin factors in the regulation of iron absorption. Res Exp Med (Berl). 1977;171:243-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 0.7] [Reference Citation Analysis (0)] |