Published online Feb 14, 2007. doi: 10.3748/wjg.v13.i6.916
Revised: December 5, 2006
Accepted: January 5, 2007
Published online: February 14, 2007
AIM: To ascertain clinical outcome and complications of self-expandable metal stents for endoscopic palliation of patients with malignant obstruction of the gastrointestinal (GI) tract.
METHODS: A retrospective review was performed throughout August 2000 to June 2005 of 53 patients with gastric outlet obstruction caused by stomach cancer. All patients had symptomatic obstruction including nausea, vomiting, and decreased oral intake. All received self-expandable metallic stents.
RESULTS: Stent implantation was successful in all 53 (100%) patients. Relief of obstructive symptoms was achieved in 43 (81.1%) patients. No immediate stent-related complications were noted. Seventeen patients had recurrent obstruction (tumor ingrowth in 14 patients, tumor overgrowth in 1 patient, and partial distal stent migration in 2 patients). The mean survival was 145 d. Median stent patency time was 187 d.
CONCLUSION: Endoscopic placement of self-expandable metallic stents is a safe and effective treatment for the palliation of patients with inoperable malignant gastric outlet obstruction caused by stomach cancer.
- Citation: Kim TO, Kang DH, Kim GH, Heo J, Song GA, Cho M, Kim DH, Sim MS. Self-expandable metallic stents for palliation of patients with malignant gastric outlet obstruction caused by stomach cancer. World J Gastroenterol 2007; 13(6): 916-920
- URL: https://www.wjgnet.com/1007-9327/full/v13/i6/916.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i6.916
Malignant obstruction of the stomach is a preterminal event that causes nausea, vomiting, dysphagia, and nutritional deficiencies leading to progressive deterioration in a patient’s quality of life. It is generally managed by surgical intervention. Because a subgroup of these patients consists of people who are elderly or have advanced, metastatic disease or other medical illness, surgery may be impossible in such patients.
Endoscopic placement of self-expandable metallic stents (SEMS) has been used for palliative treatment of patients with malignant obstruction of the gastrointestinal (GI) tract for over a decade[1,2]. Endoscopic stent placement is a safe and feasible treatment option for patients with malignant gastric outlet obstruction. In retrospective comparative studies, patients who underwent an open gastrojejunostomy had delayed oral intake after surgery[3], higher procedure-related morbidity, higher 30-d mortality[4], and a longer length of hospital stay[4-6], and incurred greater hospital charges[5] than patients who underwent an endoscopic stent insertion. To date, there has been no study that assesses clinical outcome after SEMS insertion for malignant gastric outlet obstruction caused by stomach cancer only, rather than by pancreatic, biliary, or duodenal cancer. Here we report results from a 5-year, single-center experience with the use of SEMS to palliate patients hospitalized with gastric outlet obstruction because of inoperable gastric cancer.
Between August 2000 and June 2005, 103 patients with malignant gastroduodenal and jejunal stenosis underwent endoscopic procedures with uncovered SEMS. Obstruction was caused by stomach cancer in 53 (51.3%) patients, by recurrent malignant obstruction after gastric surgery in 20 (19.4%), by pancreatic cancer in 14 (13.6%), by bile duct cancer in 7 (6.8%), by duodenal cancer in 3 (2.9%), by ampullary cancer in 2 (2.0%), by gallbladder cancer in 2 (2.0%) and unclassified in 2 (2.0%) patients. Only patients with malignant obstruction due to stomach cancer were included in this study. The diagnosis of malignant tumor and gastric outlet obstruction was established by means of endoscopy, duodenography and computed tomography. Fifty three patients (35 men, 18 women; mean age 64.0 years, range 34-85 years) with malignant gastric outlet obstruction who were treated with SEMS were followed at our outer-patient department, inpatient department or by phone calls. None of the patients were operative candidates based on the presence of advanced, metastatic disease or medical comorbidity. All patients had symptomatic obstructions such as nausea, vomiting, bloating, and abdominal pain. The degree and site of the stenosis were usually evaluated before endoscopy, using radiographs taken after oral contrast opacification; the gastric outlet area was divided into the antropyloric portion (40 patients), pyloric and duodenal bulb portion (8 patients), body and antropyloric portion (5 patients). Patient demographics are summarized in Table 1.
| Total number of patients | 53 |
| Mean age, yr ( range) | 64.0 (34-85) |
| Male/Female | 35/18 |
| Present illness | |
| Stomach cancer | 53 (100%) |
| Location of obstruction | |
| Antropyloric | 40 (75.5%) |
| Pylorus and duodenal bulb | 8 (15.1%) |
| Body and antropyloric | 5 (9.4%) |
The stents used in this study were the SEMS (NiTi-S®, Pyloric(TTS), Taewoong, South Korea), 18 mm in diameter and 60, 80, 100, or 120 mm in length. These stents are constructed from a nickel-titanium alloy with or without an outer membrane. The covered stent is coated with polyurethane in the body and the proximal flare portion. The diameters are 18 mm and 26 mm in the body and flare portions, respectively. The stent is tightly mounted on a delivery system with an outer diameter of 10-11 Fr and an overall length of 180 cm. This long and slim delivery system allowed us to insert and deploy the stent through the working channel of the therapeutic endoscope. A 300-cm long, 0.035- or 0.025-inch diameter biliary guidewire (Zebra, Microvasive, USA, or Metro, Wilson Cook, USA) or a 260-cm long, 0.035-inch diameter exchange guidewire (Radiofocus M, Terumo, Tokyo, Japan) was used. The endoscopes used were a 2-channel endoscope with a working channel of 3.7 mm (GIF-2T200, Olympus Co., Japan) or a therapeutic duodenoscope with a working channel of 4.2 mm (TJF 200, Olympus Co., Japan).
We placed all stents under endoscopic and fluoroscopic guidance. Stent insertion was performed under conscious sedation using titrated doses of midazolam administrated by an experienced endoscopist or a nurse with appropriate monitoring. After identification of the stenosis, a guidewire was passed through it using a standard endoscopic retrograde cholangiopancreatography (ERCP) catheter. The length of the stenosis was determined by the stricture identified with a water-soluble, iodinated contrast medium. The stent chosen extended at least an additional 1-2 cm in length on each side of the stenosis to allow an adequate margin of stent. An undeployed SEMS delivery system was passed through the working channel of the endoscope over the guidewire so that the ends of the undeployed stent were equidistant from the ends of the stenosis. The stent was deployed from the distal end with frequent repositioning of the proximal position in the desired location because of a tendency for it to move away from the scope. The adequacy of stent placement was assessed at the conclusion of each procedure using a combination of endoscopy and fluoroscopy. An oral contrast opacification was obtained immediately after the procedure. Technical endoscopic success was defined as correct placement of the stent across the stricture with an established patency confirmed fluoroscopically. Uncovered stents were used primarily in all cases. The patients provided informed consent before endoscopic stent insertion.
Oral intake was allowed immediately after the procedure, beginning with liquid followed by a semisolid or soft diet, if possible. When uncovered stents were occluded by tumor ingrowth, a covered stent was reimplanted through the first stent to palliate tumor ingrowth through openings between the mesh. A good clinical outcome was defined as an immediate improvement in the patient’s oral intake, thereby obviating the need for other palliative options. Follow-up investigations were performed only if symptoms recurred.
Survival was calculated using the Kaplan-Meier estimator. The date of censoring was the date of death. Cumulative stent patency was also tabulated using Kaplan-Meier plots. Censored data were determined by patients without stent occlusion and still alive, while events were determined by patients with stent occlusion, dislocation, or death.
Stent implantation was successful in all 53 patients, and we used uncovered stents initially to prevent migration. A complementary hydrostatic dilation of the stent was not needed.
Relief of major clinical symptoms (nausea, abdominal distension, reflux symptoms, vomiting) was achieved in 43 of 53 (81.1%) patients. A total of 4 of 43 (9.3%) patients could eat solid food, 25 patients (58.1%) tolerated a soft diet, and 14 patients (32.6%) continued to take liquids alone. Of the 53, 10 (18.9%) patients had intermittent episodes of vomiting, and their diets were modified as necessary (Table 2).
| Technical success, n (%) | 53 (100) |
| Clinical success, n (%) | 43 (81.1) |
| Tolerated diet, % | 43 (81.1) |
| Regular diet, % | 9.3 (4/43) |
| Soft diet | 58.1 (25/43) |
| Liquid diet | 32.6 (14/43) |
| 30-d mortality, n (%) | 10 (18.9) |
| Reintervention, n (%) | 17 (32.1) |
| Tumor ingrowth | 14 (26.4) |
| Tumor overgrowth | 1 (1.9) |
| Stent migration | 2 (3.8) |
| Mean follow up period, d | 145 (4-718) |
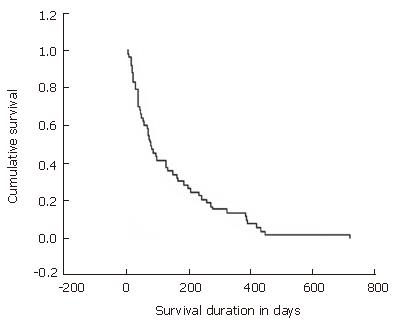
| Mean survival, d (SD) | 145 (± 150.4) |
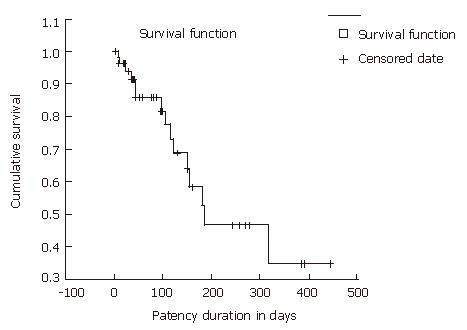
| Mean stent patency, d (range) | 187 (33-335) |
During the stent-insertion procedure, no major complications such as serious bleeding, bowel perforation, infection, or procedure-related mortality were noted. After the insertion of the stent, only minor complications such as mild abdominal pain occurred. During follow-up, stent-related problems required treatment in 17 patients (32.1%). Distal stent migration occurred partially in 2 patients (3.8%), 36 and 149 d after stent insertion, respectively, with resultant recurrence of obstructive symptoms. A second stent was inserted to overlap the first, which resulted in immediate relief of symptoms. Stent obstruction caused by proximal and distal tumor overgrowth occurred in 1 patient (1.9%) at 331 d after deployment. Tumor ingrowth through the stent mesh was observed in 14 patients (26.4%) at a mean of 78.4 d after the initial procedure. We defined early restenosis as a stent obstruction from tumor ingrowth within 4 wk after first stent implantation, and identified its development in 5 of 43 (11.6%) patients. Occlusion was identified clinically, radiologically, and endoscopically. We reimplanted covered stents in those who had tumor ingrowth or overgrowth.
The mean follow-up period for the patients was 145 d (range, 4-718 d). The mean survival was 145 d (SD; ± 150.4 d) (Figure 1), and median stent patency time was 187 d (95% CI, 39-335) (Figure 2). The overall 30-d mortality was 10 of 53 patients (18.9%).
Malignant gastric outlet obstruction is a distressing complication of gastric cancer that results in inexorable deterioration of patient quality of life. Although surgical palliation is an available option in such patients, the results of palliative gastric bypass surgery are poor with high rates of morbidity and mortality[6-8]. Because of the limitations of surgery and because many patients are elderly, frail, and in an advanced stage of disease, various techniques for restoration of bowel function by non-surgical means (eg, balloon dilation and laser photoablation) have been proposed; all have met with limited success[9,10].
Since the endoscopic application of SEMS to malignant gastric outlet obstruction in the early 1990s, the use of SEMS to pass through an obstructing neoplasm with minimal morbidity has become increasingly widespread. SEMS insertion becomes popular for treatment of malignant gastric outlet obstruction because it provides prolonged patency and simple, safe, and effective palliation in these patients.
However, the results of previous studies suggested limitations in stent patency and overall survival; these studies included heterogeneous patient groups with different causes of gastric outlet obstruction or using different types of stents. To explore the effect and complications of pyloric stents in patients with stomach cancer, we excluded patients with gastric outlet obstruction resulting from other types of cancer. To date, this is the first and largest study of endoscopic stenting for malignant gastric outlet obstruction resulting from stomach cancer only.
The results of the present study are similar or superior to those of prior published studies of enteral stent insertion for malignant gastric outlet obstruction with respect to technical success[11-15]. When we had difficulties performing the procedure, we modified the stenting technique, including using a more hydrophilic Terumo guidewire, stiffer guidewire, or a therapeutic duodenoscope; these modifications helped us overcome difficulties and achieve successful stenting. In our study, enteral stent placement allowed 81.1% of patients to resume an oral diet, an outcome similar to that reported in other studies[11,12,14-16]. The complications related to stent insertion in the present study are less than those noted in previous studies[12-14].
Restenosis by tumor ingrowth is the most common problem of uncovered stents, and some studies describe the use of covered stents to prevent this complication. Despite their ability to prevent tumor ingrowth, covered stents have been reported to migrate more often (more than 20%) than uncovered stents[17,18]. Stent migration could cause bleeding, perforation, or obstruction and require operation. Recently, a new double-layered combination stent (outer uncovered and inner covered dual stent, a Combi pyloric stent) was developed to prevent tumor ingrowth and migration and prolong stent patency in patients with gastric outlet obstruction. More data are needed to confirm the above results. Based on these results, we used uncovered stents initially. Recurrent stenosis of the stent because of progressive tumor ingrowth was a problem because most of the stents used were uncovered. Tumor ingrowth occurred in 14 (26.4%) patients at a mean of 78.4 d in our study. Overall recurrent stenosis rates of 8%-46% at an interval of 2-21 wk (mean, 7.5 wk) have been reported in other studies[14,15,19-24]. It is difficult to compare our results with results of other studies because other reports included patients with a variety of cancers. Early restenosis within 4 wk after stenting occurred in 5 (11.6%) patients in the current study. This result is similar to our previous result (14.3% for total gastric outlet obstruction patients and 10% for stomach cancer patients) and lower than our results for patients with recurrent malignant obstruction after gastric surgery (60%, unpublished data). Obstruction by tumor ingrowth usually is managed with placement of additional covered stents through the original stent.
Stent patency is influenced partly by tumor ingrowth and survival, which are also influenced by disease type. Survival is influenced by underlying diseases also. Our results of mean patient survival time and median stent patency time are somewhat longer than previously reported results[2,5,13-15], however, as stated, comparisons are difficult because ours is the sole report addressing gastric outlet obstruction resulting from stomach cancer only. Thus, the reason for these differences is not clear, but the primary illnesses that caused the gastric outlet obstruction can be the most important factor. In most western reports, the most common primary illness related to gastric outlet obstruction is pancreatic cancer, and most gastric outlet obstruction develops in the terminal stage in patients with pancreatic or biliary cancer. While in our report, stomach cancer was the sole primary disease. When compared with surgical palliation, endoscopic stenting decreases the length of hospital stay and improves survival and oral intake.
All published series on SEMS in the upper gastrointestinal tract are retrospective. The studies all differ in stent types used, and the populations differ with regard to the demographic characteristics and etiologic causes of the upper GI obstruction. There are no exact criteria regarding the selection of an uncovered stent versus a covered stent; studies are retrospective with varying definitions of complications; and a variety of stent types and insertion methods are used. Although our study is also retrospective with several similar limitations, it includes a more homogeneous patient group compared to previous studies.
In conclusion, SEMS placement is a safe and effective treatment for the palliation of patients with inoperable malignant gastric outlet obstruction caused by stomach cancer. Therefore, it should be considered as the primary choice over palliative resection for the palliation of obstruction in such patients.
S- Editor Liu Y L- Editor Zhu LH E- Editor Ma WH
| 1. | Baron TH. Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med. 2001;344:1681-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 281] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 2. | Baron TH, Harewood GC. Enteral self-expandable stents. Gastrointest Endosc. 2003;58:421-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Maetani I, Tada T, Ukita T, Inoue H, Sakai Y, Nagao J. Comparison of duodenal stent placement with surgical gastrojejunostomy for palliation in patients with duodenal obstructions caused by pancreaticobiliary malignancies. Endoscopy. 2004;36:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Wong YT, Brams DM, Munson L, Sanders L, Heiss F, Chase M, Birkett DH. Gastric outlet obstruction secondary to pancreatic cancer: surgical vs endoscopic palliation. Surg Endosc. 2002;16:310-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Yim HB, Jacobson BC, Saltzman JR, Johannes RS, Bounds BC, Lee JH, Shields SJ, Ruymann FW, Van Dam J, Carr-Locke DL. Clinical outcome of the use of enteral stents for palliation of patients with malignant upper GI obstruction. Gastrointest Endosc. 2001;53:329-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 148] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Monson JR, Donohue JH, McIlrath DC, Farnell MB, Ilstrup DM. Total gastrectomy for advanced cancer. A worthwhile palliative procedure. Cancer. 1991;68:1863-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Smith JW, Brennan MF. Surgical treatment of gastric cancer. Proximal, mid, and distal stomach. Surg Clin North Am. 1992;72:381-399. [PubMed] |
| 8. | Lillemoe KD, Barnes SA. Surgical palliation of unresectable pancreatic carcinoma. Surg Clin North Am. 1995;75:953-968. [PubMed] |
| 9. | Moses FM, Peura DA, Wong RK, Johnson LF. Palliative dilation of esophageal carcinoma. Gastrointest Endosc. 1985;31:61-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Suzuki H, Miho O, Watanabe Y, Kohyama M, Nagao F. Endoscopic laser therapy in the curative and palliative treatment of upper gastrointestinal cancer. World J Surg. 1989;13:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Kim JH, Yoo BM, Lee KJ, Hahm KB, Cho SW, Park JJ, Kim SS, Park HC, Kim JH. Self-expanding coil stent with a long delivery system for palliation of unresectable malignant gastric outlet obstruction: a prospective study. Endoscopy. 2001;33:838-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Maetani I, Tada T, Shimura J, Ukita T, Inoue H, Igarashi Y, Hoshi H, Sakai Y. Technical modifications and strategies for stenting gastric outlet strictures using esophageal endoprostheses. Endoscopy. 2002;34:402-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Nassif T, Prat F, Meduri B, Fritsch J, Choury AD, Dumont JL, Auroux J, Desaint B, Boboc B, Ponsot P. Endoscopic palliation of malignant gastric outlet obstruction using self-expandable metallic stents: results of a multicenter study. Endoscopy. 2003;35:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Telford JJ, Carr-Locke DL, Baron TH, Tringali A, Parsons WG, Gabbrielli A, Costamagna G. Palliation of patients with malignant gastric outlet obstruction with the enteral Wallstent: outcomes from a multicenter study. Gastrointest Endosc. 2004;60:916-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Kim GH, Kang DH, Lee DH, Heo J, Song GA, Cho M, Yang US. Which types of stent, uncovered or covered, should be used in gastric outlet obstructions? Scand J Gastroenterol. 2004;39:1010-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Jung GS, Song HY, Seo TS, Park SJ, Koo JY, Huh JD, Cho YD. Malignant gastric outlet obstructions: treatment by means of coaxial placement of uncovered and covered expandable nitinol stents. J Vasc Interv Radiol. 2002;13:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Jung GS, Song HY, Kang SG, Huh JD, Park SJ, Koo JY, Cho YD. Malignant gastroduodenal obstructions: treatment by means of a covered expandable metallic stent-initial experience. Radiology. 2000;216:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 135] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Park KB, Do YS, Kang WK, Choo SW, Han YH, Suh SW, Lee SJ, Park KS, Choo IW. Malignant obstruction of gastric outlet and duodenum: palliation with flexible covered metallic stents. Radiology. 2001;219:679-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Feretis C, Benakis P, Dimopoulos C, Georgopoulos K, Milas F, Manouras A, Apostolidis N. Palliation of malignant gastric outlet obstruction with self-expanding metal stents. Endoscopy. 1996;28:225-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Pinto IT. Malignant gastric and duodenal stenosis: palliation by peroral implantation of a self-expanding metallic stent. Cardiovasc Intervent Radiol. 1997;20:431-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Bethge N, Breitkreutz C, Vakil N. Metal stents for the palliation of inoperable upper gastrointestinal stenoses. Am J Gastroenterol. 1998;93:643-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Nevitt AW, Vida F, Kozarek RA, Traverso LW, Raltz SL. Expandable metallic prostheses for malignant obstructions of gastric outlet and proximal small bowel. Gastrointest Endosc. 1998;47:271-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Yates MR, Morgan DE, Baron TH. Palliation of malignant gastric and small intestinal strictures with self-expandable metal stents. Endoscopy. 1998;30:266-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Soetikno RM, Lichtenstein DR, Vandervoort J, Wong RC, Roston AD, Slivka A, Montes H, Carr-Locke DL. Palliation of malignant gastric outlet obstruction using an endoscopically placed Wallstent. Gastrointest Endosc. 1998;47:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |