Published online Dec 14, 2007. doi: 10.3748/wjg.v13.i46.6191
Revised: August 25, 2007
Accepted: September 16, 2007
Published online: December 14, 2007
AIM: To investigate the function of monocytes in Crohn’s disease (CD) patients and to correlate this with disease-associated nucleotide-binding oligomerization domain-2 (NOD2) gene variants.
METHODS: Monocytes from 47 consecutively referred CD patients and 9 healthy blood donors were cultured with interleukin (IL)-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF), and stimulated with lipopolysaccharide (LPS) or muramyldipeptide (MDP), the putative ligand of NOD2.
RESULTS: We found that monocytes from CD patients differentiated in vitro to mature dendritic cells (DCs), as determined by immunophenotype and morphology. NOD2 genotype was assessed in all subjects, and we observed high CD86 expression on immature and LPS-stimulated DCs in NOD2 mutated CD patients, as compared with wtNOD2 CD patients and controls. By contrast, CD86 expression levels of DCs induced to maturity with MDP derived from NOD2-mutated subjects were comparable to those of normal subjects. The amount of IL-12p70 in patient-cell cultures was larger than in controls after LPS treatment, but not after treatment with MDP.
CONCLUSION: Our results suggest that DCs obtained from patients with mutations in the NOD2 gene display an activated phenotype characterized by high CD86 expression, but have a diminished response to MDP when compared to the terminal differentiation phase. We speculate that the altered differentiation of monocytes might lead to an imbalance between inflammation and the killing ability of monocytes, and may be relevant to the pathogenesis of CD.
- Citation: Granzotto M, Fabbro E, Maschio M, Martelossi S, Quaglia S, Tommasini A, Presani G, Ventura A. Heterozygous nucleotide-binding oligomerization domain-2 mutations affect monocyte maturation in Crohn’s disease. World J Gastroenterol 2007; 13(46): 6191-6196
- URL: https://www.wjgnet.com/1007-9327/full/v13/i46/6191.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i46.6191
Crohn’s disease (CD) is a chronic inflammatory disorder of the gastrointestinal tract, influenced by both environmental factors and genetic predisposition[1-3]. A significant advance in the understanding of its pathogenesis was achieved by the identification of nucleotide-binding oligomerization domain-2 (NOD2) as the first susceptibility gene for CD in Caucasian populations[4,5]. This was the demonstration that a frameshift mutation (1007fs) and two nucleotide polymorphisms (R702W and G908R) in the coding region of NOD2 predispose people to the disease. Since the identification of NOD2 mutations associated with CD, much attention has been given to the function of monocytes in which this gene is constitutively expressed[6-8].
The idea that the NOD2 protein is involved in the induction of nuclear factor-kappa B (NF-κB) pro-inflammatory signaling pathways in response to bacterial infections may be relevant for understanding the role of intestinal bacteria in CD[9-12]. Indeed, functional assays on CD-associated isoforms of NOD2 have yielded controversial results concerning the production of inflammatory cytokines and responses to bacteria. NOD2 has been shown to act as both an inducer and a regulator of NF-κB and cytokine production[13,14]; more precisely, muramyldipeptide (MDP)-induced activation of NF-κB lacks mononuclear cells in CD patients homozygous for the 1007fs mutation[15]. Conversely, interleukin (IL)-12 production induced by toll-like receptor 2 (TLR2) is negatively regulated in mice by MDP co-stimulation of NOD2; although this effect is absent in NOD2-/- mice[14]. Moreover, cells obtained from knock-in mice for the 3020insC NOD2 mutation show enhanced NF-κB activity, as well as increased production of IL-1β after MDP stimulation[10]. It is noteworthy that this pro-inflammatory phenotype is also associated with an impaired response to Listeria monocytogenes challenge[13]. These data show that CD patients’ lymphomonocytes have a defect in the stress-induced production of IL-8, which may be responsible for the impaired response to bacteria[16,17].
Taken together, these data suggest a complex pathogenic model of CD, in which genetic factors favor an imbalance between the inflammatory response and the killing of mucosal bacteria. This is similar to the picture observed in some primary immunodeficiencies. Indeed, a histological lesion typical of CD, chronic granuloma, is common also in some deficiencies of the phagocytic immune system such as chronic granulomatous disease, congenital neutropenia and Wiskott-Aldrich syndrome[18,19]. Based on this, granulocyte-macrophage colony-stimulating factor (GM-CSF) has been beneficially used in patients affected by CD, probably through strengthening their natural immunity[20-22], although it shows no direct anti-inflammatory activity.
We thus hypothesized that, besides the inflammatory response itself, the function of the monocyte-derived immune system may be impaired in CD patients because of mutations in the NOD2 gene. Therefore, we looked for possible defects in monocyte differentiation in CD patients and the relationship with the NOD2 genotype.
The subjects in this study were 47 patients consecutively referred to our institute for CD, 28 males and 19 females, with a mean age of 16.6 (range 4-33) year, and a mean age at diagnosis of 12.8 year (range 1 mo-18 year). All 47 CD patients were sporadic cases. Thirty had active disease and 17 had clinical, echographic and endoscopic remission at the time of analysis. For NOD2 genotyping, a control group of 69 blood donors was analyzed. For the functional study of monocytes, the control group was 9 healthy adult blood donors (5 males and 4 females, mean age 24.3 years, range 21-38) who tested negative for CD-associated NOD2 variants. The study was approved by our local independent ethics committee, and informed consent was obtained from all patients (or their parents) and blood donors.
Patients were genotyped for R702W and G908R mutations (identified by PCR amplification and enzymatic digestion) and for the 1007fs mutation (analyzed by amplification and sequencing). DNA was extracted from peripheral blood of patients and controls using a Genomix kit (Talent, Italy). PCR reactions were performed using specific primers for the three mutations (sequences are shown in Table 1), Taq polymerase (Amplitac Gold, Applied Biosystems, Foster City, CA, USA) and a thermal cycler Gene Amp 9700 (PE Applied Biosystems). After denaturing at 95°C for 10 min, amplification was obtained after 35 cycles at 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s. For just the 1007fs mutation, 10 amplification cycles at 95°C for 30 s, 53°C for 30 s, and 72°C for 30 s, followed by another 35 cycles at 95°C for 30 s, 53°C for 30 s (touch down step: decrease of 0.5°C/cycle), and 72°C for 30 s. A final step at 72°C for 7 min was used to stop the reactions. A restriction enzyme digestion assay was performed to detect both R702W and G908R using Msp1 and HhaI, respectively. After digestion, the presence of a wild-type allele resulted in an intact fragment, whereas the variant was characterized by two bands. PCR reaction products underwent electrophoresis on 1.5% agarose gels and were visualized by ethidium bromide staining. Sequencing was carried out for 1007fs detection. Reactions were performed with a Big Dye Terminator Cycle Sequencing Ready Reaction kit (PE Applied Biosystems) and on an ABI PRISM 3100 Sequence Detector. Subjects with at least one heterozygous CD-associated variant were categorized as mtNOD2, while patients with a homozygous wild type NOD2 sequence were categorized as wtNOD2.
| Primers | Sequence |
| Arg702W (forward) | 5’-GGCGCCCCTGGAATTC-3’ |
| Arg702W (reverse) | 5’-CCTCACCCGGTGCAGC-3’ |
| Gly908Arg (forward) | 5’-CCCAGCTCCTCCCTCTTTC-3’ |
| Gly908Arg (reverse) | 5’-AAGTCTGTAATGTAAACGCCAC-3’ |
| Leu1007fsinsC (forward) | 5’-GAATGTCAGAATCAGAAGGG-3’ |
| Leu1007fsinsC (reverse) | 5’-GTCTCACCATTGTATCTTCTTTTC-3’ |
To generate ex vivo dendritic cells (DCs) from patients and controls, mononuclear cells were isolated by Ficoll separation density-gradient centrifugation, resuspended at a concentration of 2-5 × 106 cells/mL in complete RPMI-1640 medium containing 0.1% fetal calf serum (FCS), and allowed to adhere to the well surface of 24-well flat bottom plates (Corning, New York, USA) for 30 min at 37°C in a 5% CO2 incubator[23]. After washing twice with PBS to remove non-adherent cells, monolayer cells were cultured for DC differentiation in 0.5 mL RPMI-1640 supplemented with 10% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, 500 ng/mL GM-CSF (Strathmann Biotec AG, Germany), and 500 ng/mL IL-4 (Strathmann Biotec). After a 3-d culture, 100 μL medium was replaced with a fresh one containing the above-mentioned cytokines. Cell morphology was monitored by light microscopy. Analysis of cell surface marker expression was performed on a suspension of cells harvested on d 8.
On d 6 of culture, immature DCs were further maturated by adding either 100 ng/mL lipopolysaccharide (LPS; Sigma-Aldrich, Italy) plus 500 ng/mL interferon-γ (INF-γ; Strathmann Biotec) or 500 ng/mL MDP (Sigma-Aldrich) plus 500 ng/mL INF-γ for two more days. After 48 h stimulation, cells were harvested and analyzed by flow cytometry.
Cell surface marker expression was evaluated by triple immunofluorescence staining with the following monoclonal antibodies: anti-CD80-FITC, anti-CD86-PE, anti-CD83-PE, anti-CD1a-PE, anti-CD33-TC (Caltag Laboratories, Burlingame, CA, USA), anti-CD14-FITC (clone TUK4; Dako Cytomation, Denmark) and anti-HLADR FITC (Becton Dickinson, San Jose, CA, USA). Samples were acquired using a FACScan flow cytometer (Becton Dickinson) and data analysis was performed using CellQuest software (Beckton Dickinson, San Jose, CA, USA). A total of 5000 events were analyzed for each sample.
Supernatants of cell cultures were harvested and stored at -80°C until measurement of cytokines. Production of IL-12p70 was quantified using an ELISA (Bender MedSystems, Burlingame, CA, USA) according to the manufacturer’s instructions.
The t test was used to evaluate significant differences. Statistical analysis was performed with the GraphPad Prism program (San Diego, CA, USA), and P < 0.05 was considered significant.
Seventeen of the 47 CD patients were heterozygous for NOD2 allelic variants associated with CD, and 3 were double heterozygous for two mutations. The allelic frequencies of R702W, G908R and 1007fs NOD2 variants in CD patients and healthy donors are shown in Table 2. There was no correlation in our series between disease activity, pharmacological treatment, inflammatory localization and NOD2 genotype. The mean Crohn’s Disease Activity Index (CDAI) and pharmacological treatments are summarized in Table 3.
| CD (n = 47) | Healthy controls (n = 69) | |
| Genotype | ||
| Hz | 17/47 (36.1%) | 4/69 (5.8%) |
| Double Hz | 3/47 (6.4%) | 0 |
| mtNOD2 | 20/47 | 4/69 |
| wtNOD2 | 27/47 | 0/69 |
| Allelic frequencies | ||
| R702W | 5.30% | 1.45% |
| G908R | 7.45% | 0.75% |
| 1007fs | 8.50% | 0.75% |
| mtNOD2 (n = 20) | wtNOD2 (n = 27) | |
| CDAI | 24.5 | 25.2 |
| Active disease | 14 | 16 |
| Steroids | 7 | 9 |
| Methotrexate | 1 | 1 |
| Azathioprine | 4 | 5 |
| Aminosalicylic acid | 6 | 8 |
| Salazopyrine | 4 | 4 |
| Infliximab | 1 | 1 |
| Thalidomide | 4 | 5 |
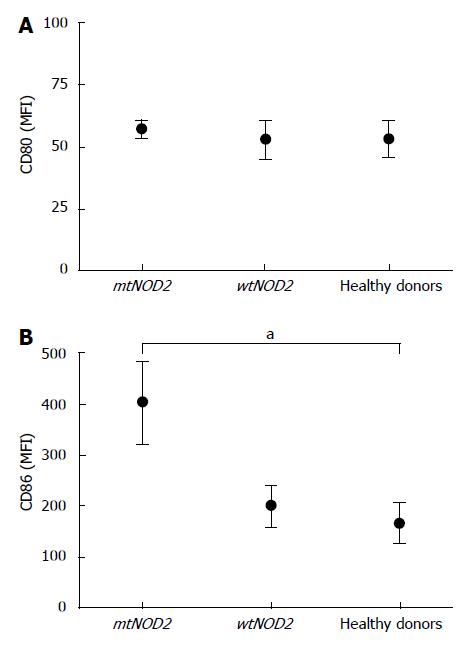
In order to obtain immature DCs, monocytes were first cultured with IL-4 and GM-CSF for 6 d and analyzed for the expression of the two co-stimulatory molecules CD80 and CD86. Under microscopy, the cells from CD patients and controls showed a typical dendritic morphology. Immunocytometry showed no significant differences in CD80 expression in either mtNOD2 or wtNOD2 patients as compared with controls. However, CD86 expression was higher in CD patients than in controls, although the difference was not significant; however, it tended to be much higher when mtNOD2 patients were compared to wtNOD2 patients and controls (P = 0.04) (Figure 1).
After LPS stimulation, terminal differentiation was obtained in DCs in patients and controls. Cells expressed high levels of activation/maturation markers, such as CD83, HLADR and CD1a, without any statistically significant differences among groups (Figure 2). Indeed, DCs from mtNOD2 patients tended to show higher CD83 expression levels, while those from wtNOD2 patients presented lower CD1a expression levels. MDP stimulation did not alter these results. Different behavior was shown by CD86 in mtNOD2 patients compared to wtNOD2 patients and healthy donors (Figure 3). A significantly greater up-regulation of CD86 was shown in mtNOD2 after LPS stimulation compared to after MDP stimulation (P = 0.016). This difference was completely absent in wtNOD2 and controls.
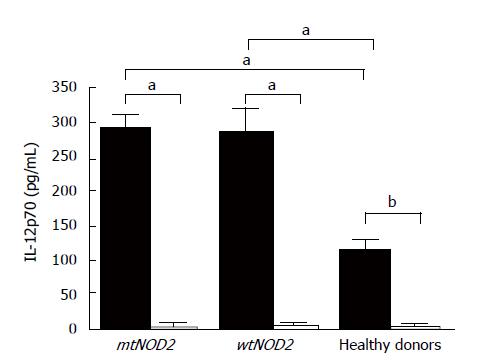
MDP and LPS stimulation of monocyte cultures from patients and healthy donors was evaluated by measuring the production of the bioactive form of IL-12p70 (Figure 4), a major regulatory cytokine of the adaptive immune response. LPS induced higher levels of cytokines in CD patients (whether mtNOD2 or wtNOD2) (mean values ± SD: 291 ± 42 pg/mL in mtNOD2 and 285 ± 61 pg/mL in wtNOD2) in comparison to controls (112 ± 38.2 pg/mL), which showed a statistically significant difference (P < 0.05). MDP, on the other hand, was inactive, thus preventing cytokine production in DCs from CD patients (mean values ± SD: 3.2 ± 7.15 pg/mL in mtNOD2 and 2.8 ± 6.2 pg/mL in wtNOD2) and healthy donors (1.94 ± 3.46 pg/mL, P = 0.0079).
Although several genes involved in CD have been described to date, the pathogenesis of the disease remains largely unknown[24,25]. It is thought that the disease arises from abnormal crosstalk between a changing intestinal flora and the host in the presence of a genetic background able to influence the integrity of the intestinal barrier and/or the functioning of the innate immune response[1,26]. Since the identification of NOD2 mutations is associated with CD, much attention has been placed on the functions of monocytes in which this gene is constitutively expressed[6]. It has been hypothesized that some slight innate immunity defects may underlie the pathogenesis of CD. Kramer et al have recently identified a defect in response to MDP stimulation of DCs obtained from patients with homozygous 3020insC NOD2 mutations, which suggests that a defect in the production of cytokines like IL-10 plays a role in the pathogenesis of the disease, by diminishing immune tolerance to intestinal bacteria.
In this work, we tested the ability of monocytes from CD patients to differentiate in vitro into DCs. The results indicated that monocytes differentiated into DCs using conventional stimuli. However, DCs obtained from CD patients with mtNOD2 showed some differences in their expression of activation antigens before and after stimulation. These differences are not likely to depend on disease activity or drugs, as the NOD2 genotype did not influence such aspects in our study (as in other published series). The most striking difference is the elevated expression of CD86 on immature mtNOD2 DCs. Moreover, the mtNOD2 group displayed a greater difference in CD86 up-regulation after LPS as compared to MDP stimulation. This was partially in agreement with the observations of Kramer et al[27] in patients with a homozygous 3020insC NOD2 mutation, whose DCs failed to up-regulate CD80 and CD86 upon MDP stimulation. In our experiment, it was of particular interest that the expression of these activation markers was higher in immature cells, thus suggesting a continuous stimulation of these cells in vivo. Indeed, a high expression of activation markers on peripheral blood monocytes from CD patients has been reported[28]. Moreover, it is noteworthy that we could see these differences in heterozygous mtNOD2 subjects when compared to wtNOD2 CD patients and controls. This suggests some interference of mutated and normal proteins, perhaps in the process of homodimerization[29].
In conclusion, we showed that DCs obtained from patients with mutations in NOD2 tended to be more activated than those obtained from wtNOD2 and controls. However, during terminal differentiation, these DCs were less responsive to MDP compared to LPS. This may be the cause of an imbalance between inflammation and the killing ability of monocytes that may be relevant to the pathogenesis of CD.
Crohn's disease (CD) is a chronic inflammatory disorder of the gastrointestinal tract. The incidence of the disease is rising in countries with improving socio-economic conditions. Although several hypotheses have been raised about the environmental factors involved in the risk of CD, the issue remains unresolved. Genetic data have recently identified genes responsible for susceptibility to CD and provided new tools for studying interactions between the immune system and the environment in the pathogenesis of CD.
Several lines of evidence suggest that the disease may arise from an altered sensing of the microbial environment in the gut by the innate immune system. (1) CD patients produce antibodies against common intestinal commensal microbes. (2) Elemental diet is an effective treatment for CD. (3) Variants of the NOD2 gene, which is involved in control of inflammatory responses to bacteria in monocytes, confer susceptibility to CD. (4) An inflammatory disease of the gut is typical of most primary immunodeficiencies involving the innate system. (5) Granulocyte monocyte-colony stimulating factor (GM-CSF) has been shown to ameliorate CD. However, the study of NOD2 mutants has brought controversial results regarding the interpretation of CD pathogenesis. The study of the behavior of CD monocytes can help clarify this issue.
Most previous studies have analyzed NOD2 mutations in cellular models, and have concluded that the consequences of NOD2 mutation are either pro-inflammatory (activation of nuclear factor-κB) or a deficiency in response to bacteria. However, the situation in vitro is more complex. We demonstrated abnormal behavior of monocytes from CD, with an easier capacity to become activated but with only minor ability to complete differentiation. While other studies have shown a defective differentiation only for NOD2 homozygous patients, we demonstrated that some differences may be present also in heterozygous patients. We hypothesized that the altered differentiation of monocytes might lead to an imbalance between inflammation and the killing ability of monocytes, and therefore is probably relevant to the pathogenesis of CD.
This study can help in the understanding of the therapeutic paradox of a disease that can be treated both with anti-inflammatory drugs and with cytokines able to strengthen the innate immune response. Further studies will be needed to determine those CD patients who are more likely to receive benefits from these two different treatment options.
wtNOD2 represents the form of the gene without mutations. DCs are the most effective cells in presenting antigens to and stimulating T cells. They can develop from monocytes and histiocytes. Muramyldipeptide (MDP) is the minimal bioactive peptidoglycan motif common to all bacteria, and it is the essential structure required for adjuvant activity in vaccines.
Generally this is a well written paper and provides further evidence concerning the effects of NOD2 mutants on immune responses to bacterial stimuli.
S- Editor Zhu LH L- Editor Kerr C E- Editor Liu Y
| 1. | Cario E. Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2. Gut. 2005;54:1182-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 234] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 2. | Augoustides JG. Inflammatory bowel disease. Lancet. 2007;370:317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Sands BE. Inflammatory bowel disease: past, present, and future. J Gastroenterol. 2007;42:16-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4223] [Cited by in RCA: 3902] [Article Influence: 162.6] [Reference Citation Analysis (0)] |
| 5. | Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3555] [Cited by in RCA: 3475] [Article Influence: 144.8] [Reference Citation Analysis (1)] |
| 6. | Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869-8872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1755] [Cited by in RCA: 1769] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 7. | Gutiérrez-Ruiz MC, Robles-Díaz G. [NOD2 gene mutation associated with susceptibility to Crohn's disease. Evidence of an alteration with links genetic and environmental factors]. Rev Invest Clin. 2001;53:386-387. [PubMed] |
| 8. | Quaglietta L, te Velde A, Staiano A, Troncone R, Hommes DW. Functional consequences of NOD2/CARD15 mutations in Crohn disease. J Pediatr Gastroenterol Nutr. 2007;44:529-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Girardin SE, Tournebize R, Mavris M, Page AL, Li X, Stark GR, Bertin J, DiStefano PS, Yaniv M, Sansonetti PJ. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2001;2:736-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 475] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 10. | Maeda S, Hsu LC, Liu H, Bankston LA, Iimura M, Kagnoff MF, Eckmann L, Karin M. Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 583] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 11. | Vignal C, Singer E, Peyrin-Biroulet L, Desreumaux P, Chamaillard M. How NOD2 mutations predispose to Crohn's disease? Microbes Infect. 2007;9:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Eckburg PB, Relman DA. The role of microbes in Crohn's disease. Clin Infect Dis. 2007;44:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 13. | Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1334] [Cited by in RCA: 1325] [Article Influence: 66.3] [Reference Citation Analysis (2)] |
| 14. | Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 602] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 15. | Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003;278:5509-5512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 1251] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 16. | Li J, Moran T, Swanson E, Julian C, Harris J, Bonen DK, Hedl M, Nicolae DL, Abraham C, Cho JH. Regulation of IL-8 and IL-1beta expression in Crohn's disease associated NOD2/CARD15 mutations. Hum Mol Genet. 2004;13:1715-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | van Lierop PP, Damen GM, Escher JC, Samsom JN, Nieuwenhuis EE. Defective acute inflammation in Crohn's disease. Lancet. 2006;368:578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Ochs HD, Ament ME, Davis SD. Structure and function of the gastrointestinal tract in primary immunodeficiency syndromes (IDS) and in granulocyte dysfunction. Birth Defects Orig Artic Ser. 1975;11:199-207. [PubMed] |
| 19. | Korzenik JR, Dieckgraefe BK. Is Crohn's disease an immunodeficiency? A hypothesis suggesting possible early events in the pathogenesis of Crohn's disease. Dig Dis Sci. 2000;45:1121-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Wilk JN, Viney JL. GM-CSF treatment for Crohn's disease: a stimulating new therapy? Curr Opin Investig Drugs. 2002;3:1291-1296. [PubMed] |
| 21. | Moss AC, Farrell RJ. Adding fuel to the fire: GM-CSF for active Crohn's disease. Gastroenterology. 2005;129:2115-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Korzenik JR, Dieckgraefe BK, Valentine JF, Hausman DF, Gilbert MJ. Sargramostim for active Crohn's disease. N Engl J Med. 2005;352:2193-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 23. | D'Amico G, Bianchi G, Bernasconi S, Bersani L, Piemonti L, Sozzani S, Mantovani A, Allavena P. Adhesion, transendothelial migration, and reverse transmigration of in vitro cultured dendritic cells. Blood. 1998;92:207-214. [PubMed] |
| 24. | Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De La Vega FM, Briggs J. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1519] [Cited by in RCA: 1453] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 25. | Török HP, Glas J, Lohse P, Folwaczny C. Genetic variants and the risk of Crohn's disease: what does it mean for future disease management? Expert Opin Pharmacother. 2006;7:1591-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Yamamoto-Furusho JK, Korzenik JR. Crohn's disease: innate immunodeficiency? World J Gastroenterol. 2006;12:6751-6755. [PubMed] |
| 27. | Kramer M, Netea MG, de Jong DJ, Kullberg BJ, Adema GJ. Impaired dendritic cell function in Crohn's disease patients with NOD2 3020insC mutation. J Leukoc Biol. 2006;79:860-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Liu ZX, Hiwatashi N, Noguchi M, Toyota T. Increased expression of costimulatory molecules on peripheral blood monocytes in patients with Crohn's disease. Scand J Gastroenterol. 1997;32:1241-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Inohara N, Nuñez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol. 2003;3:371-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 737] [Article Influence: 33.5] [Reference Citation Analysis (0)] |