Published online Nov 28, 2007. doi: 10.3748/wjg.v13.i44.5951
Revised: July 26, 2007
Accepted: October 10, 2007
Published online: November 28, 2007
We report a case of a poorly differentiated epithelial tumour of the rectum with a highly pleomorphic morphology and an aberrant immunophenotype, including the expression of epithelial markers, the focal parameter of neuroendocrine differentiation, and the unexpected detection of CD-117 overexpression. A 69-year-old man was admitted to our clinic complaining of rectal bleeding and weight loss. Colonoscopy showed an ulcerative bleeding mass located about 8 cm from the anal verge. Abdominal and pelvis CT scans demonstrated a large low-density lesion with extracanalicular growth from the middle rectum, with local lymph-node spread, and without tumour infiltration of other pelvic organs, or evidence of distant intra-abdominal spread. The patient underwent a low anterior resection for rectal cancer together with wide resection of lymph nodes. In immunohistochemical analysis, pankeratin and Epithelial Membrane Antigen (EMA) immunolabeling proved the epithelial nature of the tumor cells. Chromogranin A and Leukocyte Common Antigen (LCA) were negative, whereas CD-56 expression was scanty and Neuron Specific Enolase (NSA) was heavily and diffusely expressed. Ki67 immunoexpression was particularly increased. Interestingly, the intense c-kit immunoreactivity (100%) was a common feature. The above phenotypic and immunohistochemical profile was consistent with an anaplastic carcinoma of the large intestine, with focal neuroendocrine differentiation and diffuse immunoreactivity to c-kit protein. Given the resistance of this tumor to conventional chemotherapy and radiation, the incidence of the c-kit alteration may represent a novel approach to a gene-directed treatment using a c-kit inhibitor (STI571) similar to that which has been proposed in GISTs.
- Citation: Giannopoulos A, Papaconstantinou I, Alexandrou P, Petrou A, Papalambros A, Felekouras E, Papalambros E. Poorly differentiated carcinoma of the rectum with aberrant immunophenotype: A case report. World J Gastroenterol 2007; 13(44): 5951-5953
- URL: https://www.wjgnet.com/1007-9327/full/v13/i44/5951.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i44.5951
We report a case of a poorly differentiated epithelial tumour of the rectum with a highly pleomorphic morphology and an aberrant immunophenotype, including the expression of epithelial markers, the focal parameter of neuroendocrine differentiation, and the unexpected detection of CD-117 overexpression.
A 69-year-old man was admitted to our clinic complaining of rectal bleeding for 2 mo (two episodes of massive rectal bleeding) and weight loss of 5 kg in 4 mo. His past medical history was negative for any surgical procedure or chronic disease, and his family history was also free. He denied any change in bowel habits, urinary urgency, or any other symptoms. Digital examination was normal but proctosigmoidoscopy showed an ulcerative mass bulging over the right rectal wall, and the fecal examination was positive for blood.
Laboratory tests of the peripheral blood revealed microcytic hypochromic anemia (hemoglobin, 11.7 g/dL and hematocrit, 26.6%). The serum levels of carcinoembrionic antigen (CEA), alpha-fetoprotein AFP, and CA19-9 were within normal ranges. Prostate-specific antigen (PSA) was also within the normal range (0.7 ng/dL, PSA free, 0.16 mg/dL).
Colonoscopy showed an ulcerative bleeding mass that was located about 8 cm from the anal verge. An additional abnormality revealed by colonoscopy was the existence of five small polyps along the rest of the colon. Abdominal and pelvis CT scans demonstrated a large low-density lesion with extracanalicular growth from the middle rectum, with local lymph-node spread, and without tumour infiltration of other pelvic organs, or evidence of distant intra-abdominal spread. No metastatic nodules were found in the lung and the liver by diagnostic imaging procedures. The patient underwent a low anterior resection for rectal cancer with a circular stapled low, end-to-end colorectal anastomosis (indicated for tumours situated 6-9 cm above the anal verge), together with wide resection of lymph nodes.
On gross examination, the 14-cm rectosigmoidal surgical specimen manifested as an ulcerative tumor that measured 5 cm in its larger diameter, located 2-5 cm from the distal resection margin.
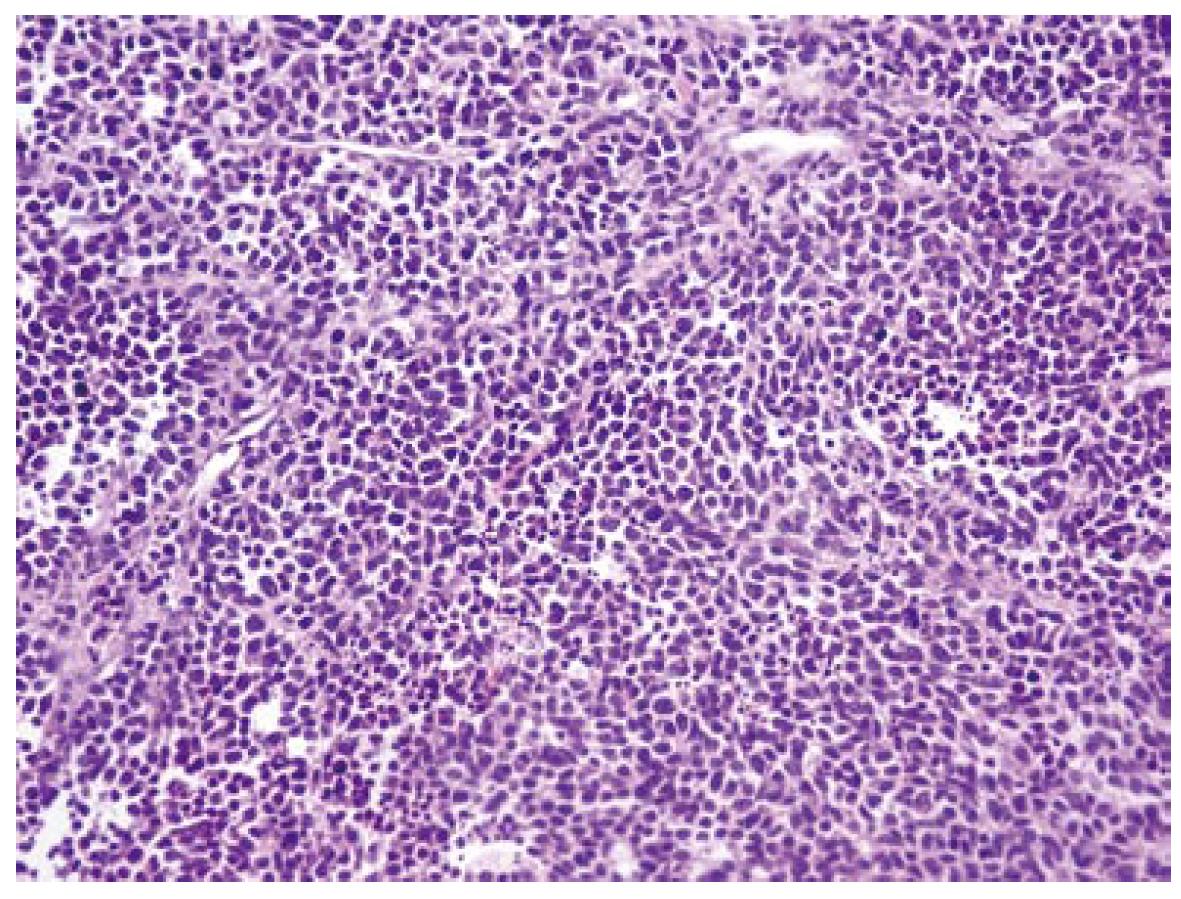
Under microscopy, the tumor was composed of irregular sheets and scattered tumor cells (Figure 1) with markedly pleomorphic nuclei and prominent nuclei, including giant or multinucleated cell types. The tumor was found to infiltrate the submucosa, the muscularis propria, and the perirectal adipose tissue. Nodal metastasis was found in 2/22 lymph nodes examined.
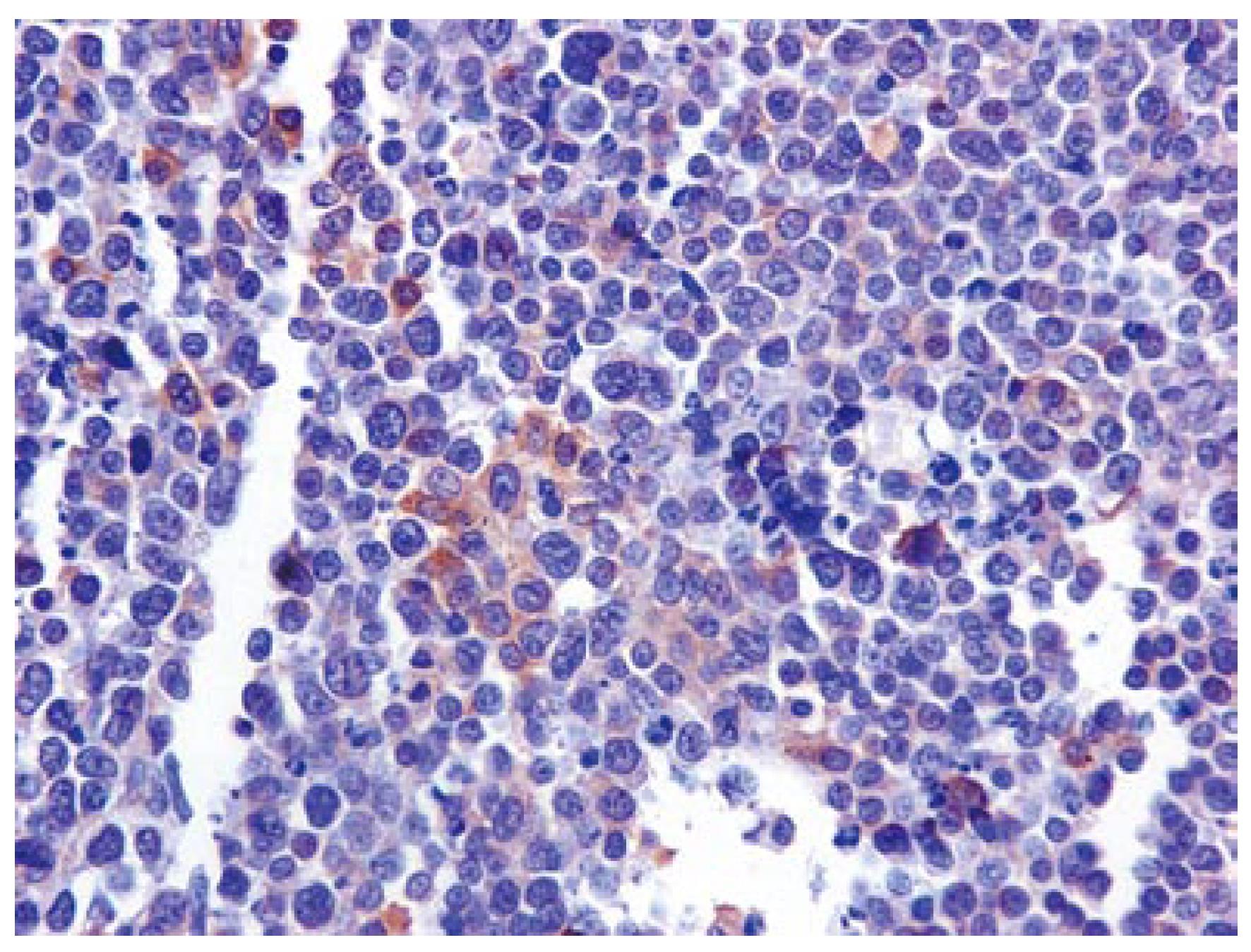
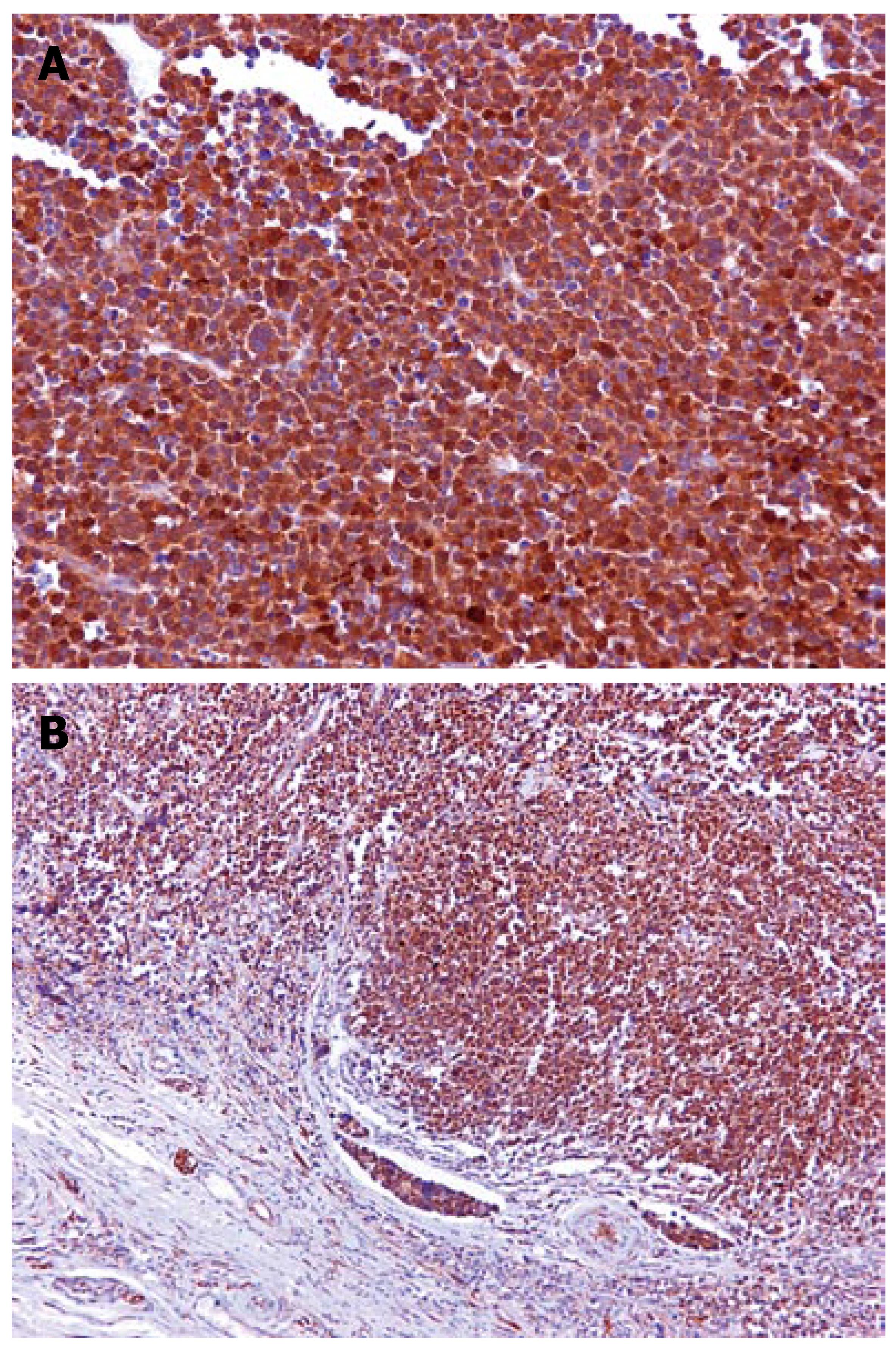
In immunohistochemical analysis, pankeratin and Epithelial Membrane Antigen (EMA) immunolabeling proved the epithelial nature of the tumor cells. Chromogranin A and Leukocyte Common Antigen (LCA) were negative, whereas CD-56 expression was scanty (Figure 2), and Neuron Specific Enolase (NSA) was heavily and diffusely expressed. Ki67 immunoexpression was particularly increased. Interestingly, the intense c-kit immunoreactivity (100%) was a common feature (Figure 3A and B). The above phenotypic and immunohistochemical profile was consistent with an anaplastic carcinoma of the large intestine, with focal neuroendocrine differentiation and diffuse immunoreactivity to c-kit protein.
c-kit protein, a 145-kDa tyrosine kinase with oncogenic properties is a transmembrane receptor growth factor known as a stem cell factor (SCF). It is encoded by the c-kit proto-oncogene located on chromosome 4q11-q12[1]. Activation of c-kit by its SCF ligand leads to dimerization of the receptor. The latter activates further signalling cascades that control cell proliferation, adhesion and differentiation[2].
CD-117 is a functionally important protein in hematopoietic stem cells, mast cells, germ cells, some epithelial cells and in Cajal cells. Parenthetically, Cajal cells are known to originate from common intestinal mesenchymal precursor cells[2-5].
Several studies have identified the presence of a c-kit malignant mutation in over half of gastrointestinal stromal tumors (GISTs), as well as in other human tumors, including germ cell tumors, neuroblastoma, melanoma, ovarian carcinoma and breast carcinoma[6-14]. Interestingly, overexpression of c-kit has been found to affect proliferation in human neural, lung, breast, colorectal, skin and prostatic tumors[15].
On the basis of an immunohistochemical study of c-kit expression in 126 colorectal carcinomas, only two (1.6%) poorly differentiated carcinomas presented with aberrant c-kit positivity, which implies the role of c-kit in tumor progression[16]. Although the functional role of mutated c-kit kinase activity is not fully understood, it seems that in breast, thyroid and ovarian cancer, the malignant transformation seems to correlate with loss of c-kit protein expression[17]. However, Bellon et al[12] have reported overexpression of c-kit in human colorectal cancer, and have suggested that c-kit activation is critical for growth, survival, migration and invasive potentional of DLD-1 colon carcinoma cells. Of interest, only 1.6% of colorectal cancers show high cytoplasmic c-kit staining, a fact that is not related definitely to tumorigenesis[16]. Immunohistochemical expression of c-kit protein is a rare event in poorly differentiated carcinomas[16,17].
In the study by Akintola-Ogunremi et al[17], who studied 66 cases of primary colorectal neuroendocrine carcinoma, the prognosis did not appear to differ between kit-positive and kit-negative cases. In the view of the limited number of reports in the literature and the lack of follow-up data, c-kit overexpression cannot provide any evidence regarding the biological behavior of the tumor currently described. However, further follow-up, together with c-kit gene mutational analysis may alter the prognostic value of c-kit positivity in these highly aggressive malignancies of the colon. Thus, the immunohistochemical CD-117 alteration in poorly differentiated carcinoma of the rectum remains to be elucidated.
Given the resistance of this tumor to conventional chemotherapy and radiation[18,19], the incidence of the c-kit alteration may represent a novel approach to a gene-directed treatment using a c-kit inhibitor (STI571) similar to that which has been proposed in GISTs[20]. According to the literature, STI571 may inhibit the in vitro growth of colorectal carcinoma cell lines, although it has not been tested so far for the treatment of colorectal carcinoma[20].
A long term study of c-kit protein expression in poorly differentiated malignancies of colon may be warranted, although c-kit overexpression can not guarantee tumor response. Thus, a thorough genetic investigation of colorectal malignancy may determine the eligibility of STI571 regimen for potential targeted therapy.
S- Editor Liu Y L- Editor Kerr C E- Editor Liu Y
| 1. | Linnekin D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int J Biochem Cell Biol. 1999;31:1053-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 269] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 2. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 3. | Bernex F, De Sepulveda P, Kress C, Elbaz C, Delouis C, Panthier JJ. Spatial and temporal patterns of c-kit-expressing cells in WlacZ/+ and WlacZ/WlacZ mouse embryos. Development. 1996;122:3023-3033. [PubMed] |
| 4. | Vannucchi MG. Receptors in interstitial cells of Cajal: identification and possible physiological roles. Microsc Res Tech. 1999;47:325-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3114] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 6. | Beck D, Gross N, Brognara CB, Perruisseau G. Expression of stem cell factor and its receptor by human neuroblastoma cells and tumors. Blood. 1995;86:3132-3138. [PubMed] |
| 7. | DiPaola RS, Kuczynski WI, Onodera K, Ratajczak MZ, Hijiya N, Moore J, Gewirtz AM. Evidence for a functional kit receptor in melanoma, breast, and lung carcinoma cells. Cancer Gene Ther. 1997;4:176-182. [PubMed] |
| 8. | Hibi K, Takahashi T, Sekido Y, Ueda R, Hida T, Ariyoshi Y, Takagi H, Takahashi T. Coexpression of the stem cell factor and the c-kit genes in small-cell lung cancer. Oncogene. 1991;6:2291-2296. [PubMed] |
| 9. | Hines SJ, Litz JS, Krystal GW. Coexpression of c-kit and stem cell factor in breast cancer results in enhanced sensitivity to members of the EGF family of growth factors. Breast Cancer Res Treat. 1999;58:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Timeus F, Crescenzio N, Valle P, Pistamiglio P, Piglione M, Garelli E, Ricotti E, Rocchi P, Strippoli P, Cordero di Montezemolo L. Stem cell factor suppresses apoptosis in neuroblastoma cell lines. Exp Hematol. 1997;25:1253-1260. [PubMed] |
| 11. | Turner AM, Zsebo KM, Martin F, Jacobsen FW, Bennett LG, Broudy VC. Nonhematopoietic tumor cell lines express stem cell factor and display c-kit receptors. Blood. 1992;80:374-381. [PubMed] |
| 12. | Bellone G, Silvestri S, Artusio E, Tibaudi D, Turletti A, Geuna M, Giachino C, Valente G, Emanuelli G, Rodeck U. Growth stimulation of colorectal carcinoma cells via the c-kit receptor is inhibited by TGF-beta 1. J Cell Physiol. 1997;172:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Simak R, Capodieci P, Cohen DW, Fair WR, Scher H, Melamed J, Drobnjak M, Heston WD, Stix U, Steiner G. Expression of c-kit and kit-ligand in benign and malignant prostatic tissues. Histol Histopathol. 2000;15:365-374. [PubMed] |
| 14. | Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, Hsu RY, Birkett NC, Okino KH, Murdock DC. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 982] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 15. | Sammarco I, Capurso G, Coppola L, Bonifazi AP, Cassetta S, Delle Fave G, Carrara A, Grassi GB, Rossi P, Sette C. Expression of the proto-oncogene c-KIT in normal and tumor tissues from colorectal carcinoma patients. Int J Colorectal Dis. 2004;19:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Reed J, Ouban A, Schickor FK, Muraca P, Yeatman T, Coppola D. Immunohistochemical staining for c-Kit (CD117) is a rare event in human colorectal carcinoma. Clin Colorectal Cancer. 2002;2:119-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Akintola-Ogunremi O, Pfeifer JD, Tan BR, Yan Y, Zhu X, Hart J, Goldblum JR, Burgart L, Lauwers GY, Montgomery E. Analysis of protein expression and gene mutation of c-kit in colorectal neuroendocrine carcinomas. Am J Surg Pathol. 2003;27:1551-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Pidhorecky I, Cheney RT, Kraybill WG, Gibbs JF. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol. 2000;7:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 316] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 19. | Pierie JP, Choudry U, Muzikansky A, Yeap BY, Souba WW, Ott MJ. The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Arch Surg. 2001;136:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 208] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Melis M, Choi EA, Anders R, Christiansen P, Fichera A. Synchronous colorectal adenocarcinoma and gastrointestinal stromal tumor (GIST). Int J Colorectal Dis. 2007;22:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |