Published online Nov 28, 2007. doi: 10.3748/wjg.v13.i44.5805
Revised: December 12, 2006
Accepted: September 15, 2007
Published online: November 28, 2007
Multidisciplinary approach for rectal cancer treatment is currently well defined. Nevertheless, new and promising advances are enriching the portrait. Since the US NIH Consensus in the early 90’s some new characters have been added. A bird’s-eye view along the last decade shows the main milestones in the development of rectal cancer treatment protocols. New drugs, in combination with radiotherapy are being tested to increase response and tumor control outcomes. However, therapeutic intensity is often associated with toxicity. Thus, innovative strategies are needed to create a better-balanced therapeutic ratio. Molecular targeted therapies and improved technology for delivering radiotherapy respond to the need for accuracy and precision in rectal cancer treatment.
- Citation: Diaz-Gonzalez JA, Arbea L, Aristu J. Rectal cancer treatment: Improving the picture. World J Gastroenterol 2007; 13(44): 5805-5812
- URL: https://www.wjgnet.com/1007-9327/full/v13/i44/5805.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i44.5805
Since the early 90’s, radical surgery and fluoropirimidine-based chemoradiotherapy (CHRT) are the gold standards of treatment for locally advanced rectal cancer. Studies conducted by the Gastrointestinal Tumor Study Group[1,2] and the North Central Cancer Treatment Group[3] concluded that the combination of postoperative chemo-therapy with radiotherapy improved local tumor control and survival in stage II and III rectal cancer relative to surgery alone.
Although currently the big picture mostly remains, some of the characters of the puzzle have changed. The main milestones in this development began with the improvement of the surgical technique, total mesorectal excision (TME). TME became the choice surgical procedure, with a relevant increase in local control. Actually, at some point it was thought that TME could make radiotherapy (RT) unnecessary. Nevertheless, a randomized study soon followed showing the maintained benefit of RT despite an excellent surgery, at least in terms of local control[4], outcomes that even are improving with longer follow-up.
The second landmark was to move the CHRT segment before the surgery. Initially, preoperative radiotherapy was found to improve overall survival as compared with surgery alone[5,6]. In the last decade, the dominant tendency in the therapeutic development of rectal cancer, both in Europe and North America, has been the use of preoperative radiotherapy with conventional protracted fractionation (45-50 Gy in daily fractions of 1.8-2 Gy during 5-6 wk) with concurrent chemotherapy followed by surgery at 4-8 wk. Extensive experience with preoperative CHRT showed feasibility and promising results in terms of down staging, sphincter preservation and disease control and survival parameters as interesting elements of analysis, with an acceptable toxicity profile. The most frequently used chemotherapy agent in this clinical context is 5-fluorouracil (5-FU, i.v.)[7-13]. More recently, the only phase III trial concluded comparing pre- vs post-operative CHRT, demonstrated better tolerance, sphincter-saving surgical procedures and local control with preoperative CHRT[14].
Preoperative radiotherapy alone (no chemotherapy) and delayed surgery reported down staging rates of 18%[15,16]. However, the prolonged administration of CH-RT achieves down staging figures of around 65%[7-11,17]. Additionally, induction of tumor down staging improves the probability of a complete resection and sphincter-preserving surgery[11,13,18-20].
Complete pathologic response (pCR) rates range from 8% to 27% using i.v. 5-FU with preoperative irradiation[7,10,11,14,21]. In studies of postoperative 5-FU-based CHRT, severe acute toxicity ranges from 24%-40%[1,14,22,23]. However, in Phase II studies of preoperative CH-RT, Grade 3-4 acute toxicity occurs in 15%-28% of patients[7,11,13,14,20].
Regarding tumor control and survival, published series vary in follow-up. Preoperative CHRT in rectal cancer assumes ranges for 5-year local recurrence from 2% to 15%, disease-free survival from 70% to 86%, and overall survival from 60% to 85%[7,9,10,14,18,21,24-26].
In summary, incorporation of TME surgical procedure and 5-FU-based preoperative CHRT have been translated to an improvement in local control, with the additional advantage of more tolerable treatments in terms of acute toxicity and saving-sphincter surgical procedures.
The picture is drawn. What is next, more characters or better colors?
Therapeutic intensity is often linked to better response and outcomes. But in oncology more is not always better. Increases in doses or number of therapeutic agents combined together lead to higher rates of toxicity. This situation is especially true in rectal cancer. Moreover, the risk of over-treatment in some patients with rectal cancer is present. One treatment approach for all rectal adjuvant patients may not be warranted. We already know that not every stage II-III rectal cancer is the same[27]. Prognostic factors have been studyed, both at clinical and at molecular and genetic level. In the near future these signatures should be taken into account. An adequate therapeutic index should be found, with a well-balanced ratio of benefit/toxicity.
Where can we find additional benefit in rectal cancer treatment? On the one hand, despite the improvement in local control with multimodality approaches, the rate of distant metastasis is still high, around 19%-36%[10,14]. On the other hand, growing data demonstrates a relationship between response to preoperative CHRT and survival. A higher grade of tumor regression in the surgical specimen has been associated with increased disease-free survival and overall survival after preoperative CHRT in rectal cancer[10,24,17,28-31]. Thus, achieving higher rates of complete pathologic response, but also major tumor regression, is one of the current goals in the protocols of preoperative CHRT in rectal cancer. Both effects, reduction of distant metastasis and higher tumor regression grade, require the use of more active and effective chemotherapeutics agents, with adequate toxicity profiles when administered with radiotherapy.
Oral fluoropyrimidines: Oral fluoropyrimidines have been developed as a therapeutic alternative to i.v., continuous infusion of 5-FU, and have been shown to deliver similar efficacy and tolerability with the additional advantage of offering the convenience of oral chemotherapy (Table 1).
| Chemotherapy | RT (Gy) | GI grade 3-4 toxicity (%) | DS (%) | pCR (%) | ||
| Capecitabine (mg/m2 bid) | ||||||
| Kim et al | 825 d 1-14 and 22 - 35 | 50.4 | - | 84 | 31 | |
| De Paoli et al | 825 bid continuous | 50.4 | - | 57 | 24 | |
| 5-FU (mg/m2 CI) | CPT-11 (mg/m2 weekly) | |||||
| Mehta et al | 200 | 50 | 50.4 | 28 | 71 | 37 |
| Klautke et al | 250 | 40 | 50.4 | 32 | 76 | 24 |
| Mohiuddin et al | Arm 1: 225 | Arm 1: - | HART: 55.2-60 | 27 | 78 | 26 |
| Arm 2: 225 | Arm 2: 50 | 50-54 | 37 | 78 | 26 | |
| Navarro et al | 225 | 50 | 45 | 14 | 49 | 14 |
| 5-FU (mg/m2 CI) | Oxaliplatin (mg/m2) | |||||
| Ryan et al | 200 | MTD: 60 weekly | 50.4 | 38 | - | 25 |
| Aschele et al | 200-225 | MTD: 60 weekly | 50.4 | 16 | 84 | 28 |
| Turrito et al | 300 | 80 wk 1, 3, 5 | 45 | - | 65 | 15 |
| Capecitabinec (mg/m2 bid) | Oxaliplatin (mg/m2) | |||||
| Rodel et al | 825 d 1-14 and 22 - 35 | 50 d 1, 8, 22 | 50.4 | 6 | 55 | 19 |
| Machiels et al | 825 bid continuous | 50 weekly | 45 | 30 | - | 14 |
Few studies have investigated the safety and efficacy of tegafur with or without uracile (5-FU pro-drugs) and radiotherapy[32-35]. Down staging rates (54%-68%), pCR (8%-15%), and grade 3-4 toxicity (12%-43%) match quite well with those with i.v. 5-FU. Although follow-up is not as long as in the 5-FU series, outcomes in terms of local control, distant metastasis rate, disease-free survival and overall survival seem to be similar.
Capecitabine is a fluoropyrimidine carbamate active in several solid tumors. A recent phase III trial (X-ACT trial) has demonstrated the equivalence of capecitabine to bolus 5-FU/leucovorin in the adjuvant treatment of colon cancer[36]. Thymidine phosphorylase (TP) is a key enzyme for the metabolism of capecitabine to 5-FU. Some data suggest that tumor tissue shows higher concentrations of TP than normal tissue[37]. This phenomenon would lead to a preferential activation of capecitabine in the tumor tissue, providing a favorable ratio for toxicity and radiosensitization. Preclinical studies have shown that RT might up-regulate the TP expression in tumor cells, resulting in a selective and synergistic effect between RT and capecitabine[38]. PhaseIstudies have been conducted to determine the maximun-tolerated-dose (MTD) of capecitabine in combination with radiotherapy. The recommended dose for this combination was 825 mg/m2 bid without break during radiotherapy period (5-6 wk)[39,40]. Two published phase II studies have shown that preoperative CHRT with capecitabine appears to be effective in locally advanced, resectable rectal cancer. Encouraging rates of down staging (up to 84%) and pCR (24%-31%) with a favorable safety profile of the combination might warrant the use of capecitabine and RT with other effective new drugs[40-42].
Irinotecan (CPT-11): Irinotecan is an active chemo-therapeutic agent in colorectal cancer. The combination of Irinotecan and 5-FU has been approved as first line chemotherapy for patients with metastatic colorectal cancer[41,43,44]. PhaseIstudies have demonstrated that CPT-11 can be safely administered concomitantly with radiotherapy (MTD: 10 mg/m2 daily or 50 mg/m2 weekly)[45]. Several phase II studies have determined the efficacy and feasibility of the irinotecan and 5-FU combined-therapy plus radiotherapy in the neo-adjuvant management of rectal cancer. The rates of tumor down staging (49%-78%) and pCR are high (14%-37%) with an acceptable rate of acute severe toxicity (14%-37%)[46-49].
The combination of CPT-11 and Capecitabine with radiotherapy has been studied in recent phaseI-II trials[50,51]. The MTD dose of Capecitabine was 500 mg/m2 while combining with CPT-11 50 mg/m2 weekly and 750 mg/m2 while combining whit CPT-11 40 mg/m2 weekly. The rate of tumor down staging and pCR were similar with the two schedules (72%-75% and 14%-21%, respectively) and similar with the combination of 5-FU, CPT-11 and radiotherapy.
Oxaliplatin: Oxaliplatin is a novel anti-neoplastic platinum. When combined with 5-FU, oxaliplatin improves overall survival for patients with metastatic colorectal cancer and the rate of progression-free survival for patients with completely resected stage II and III colon cancer[52,53]. These data encourage combining oxaliplatin and 5-FU in the preoperative setting of rectal cancer management for an improved response. Moreover, oxaliplatin has radiation sensitization properties[54].
Several phase II studies have evaluated weekly administration schedules of oxaliplatin and 5-FU and radiotherapy. They have demonstrated that this regimen is feasible with moderate toxicity. The addition of oxaliplatin to standard 5-FU-RT seems to be associated with a promising down staging (65%-84%) and pCR rates (15%-28%)[55-57].
Oxaliplatin has been combined with Capecitabine in metastatic colorectal disease[58-60]. The combination has been adapted to preoperative CHRT and phaseI-II trials have been published. The studies show that this regimen is active and feasible, with attractive down staging (55%-72%) and pCR rates (14%-28%)[61-63].
One of the paradigms for loco regional treatment of cancer is anatomic precision. Technical advances in radiation oncology including functional and molecular imaging and intensity-modulated radiation therapy (IMRT) delivery techniques are allowing greater treatment precision and dose escalation. Moreover, cancer is a biologic entity. Treating cancer requires understanding cancer biology which is changing the approach in cancer therapeutics. A number of genetic signatures and molecular pathways involved in cancer have been discovered. Parallel molecular therapeutic development is emerging. Molecular targeted treatments have being combined with conventional anticancer drugs, accordingly with specific tumor biology.
Coming back to loco regional treatment of rectal cancer, IMRT might provide anatomical specificity. Molecular therapies will complement anatomical specificity by targeting biological pathways that are deregulated in individual tumors. Precision is technologically based while accuracy is biologically based[64].
Epidermal growth factor receptor (EGFR) and angiogenesis-related pathways are perhaps the molecular mechanisms best explored in colorectal cancer. Both mechanisms are involved either in colorectal carcinogenesis and tumor growth[65,66], and in radioresistence[67-69]. Thus, novel targeted biologic agents including angiogenesis and EGFR inhibitors hold tremendous promise as RT sensitizers and as systemic therapy in rectal cancer[69-71].
Preliminary reports show feasibility and promising activity combining Bevacizumab with 5-FU and RT. The MTD was determined for Bevacizumad at 5 mg/kg[72]. Additionally, surrogate markers are being investigated suggesting the ability of Bevacizumab to specifically target tumor angiogenesis[72,73].
A recent phaseIstudy combining capecitabine, oxali-platin and bevacizumab with preoperative RT establishes the MTD to be capecitabine 625 mg/m2 BID, Oxaliplatin 50 mg/m2 per week and Bevacizumab 15 mg/kg d 1 and 10 mg/kg d 8 and 22. Down staging was observed in 9/11 patients (82%) and 2/11 (18%) patients achieved pCR and in 2 of 11 only microscopic disease was found in the surgical specimen[74].
C225 (Cetuximab) is a chimeric monoclonal antibody that targets the extracellular domain of epidermal growth factor receptor (EGFR) with high specificity and affinity[75]. Cetuximab has demonstrated increased responses combined with chemotherapy in metastasic colorectal cancer[76]. The radiosensitization activity of Cetuximab has been broadly explored[77]. Thus, the combination of chemotherapy and RT with C225 is an attractive strategy to be explored.
A pilot study has explored the addition of Cetuximab (250 mg/m2 per week) to conventional i.v., continuous infusion of 5-FU and RT. Grade 3-4 diarrhea was detected in 10% and acneiform rash in 15%. Pathological complete response was achieved in 12% of patients[78].
Cetuximab has been combined with Capecitabine and RT in rectal cancer. The dose suggested is Capecitabine 825 mg/m2 bid without interruption during the duration of RT and Cetuximab 250 mg/m2 weekly. Grade 3 diarrhea was 10%, rectal pain 20%. Ten percent of the evaluated patients achieved pCR[79].
A phaseItrail has recently evaluated the combination of Capecitabine, Oxaliplatin and C225 with RT. Doses suggested were for Cetuximab 400 mg/m2 on d-7, then 6 weekly doses of 250 mg/m2, for oxaliplatin 50 mg/m2 d 1, 8, 22 and 29 in combination with capecitabine 1650 mg/m2 bid d 1-14 and 22-35. Grade 3-4 diarrhea was 15% and grade 3-4 toxicity as skin reaction 7%[80]. The results of the phase II study with 31 patients enrolled are coming soon.
New drugs and biological treatments may enhance global radiotherapy effects improving therapeutic outcomes but acute effects may also be increased. Moreover, a dose-volume relationship has been established between the severity of diarrhea toxicity and the volume of irradiated small bowel at all dose levels in patients treated with preoperative chemoradiation for rectal cancer[81]. The volume of irradiated small bowel thresholds to predict acute gastrointestinal toxicity is unknown although a strong correlation exists between the volume of small bowel receiving 15 Gy (V15) and the degree of acute small bowel toxicity[82].
The development of novel and sophisticated irradiation techniques as intensity modulated radiation therapy (IMRT) represents a spectacular progress in planning and delivering external beam radiation therapy. IMRT generates highly conformal and irregularly shaped dose distribution while reducing dose to adjacent normal tissue structures. IMRT has demonstrated dosimetric superiority over 3D-conformal radiation therapy (3D-CRT) in the majority of tumor sites, including pelvic tumors where the irradiated bowel can be significantly reduced[83].
Researchers at the Royal Marsden Hospital have reported a dosimetric study comparing IMRT vs 3D-CRT in five rectal cancer patients. The irradiated bowel volume at 45 Gy and 50 Gy can be reduced with IMRT techniques, which could potentially resulted in marked reductions in acute and chronic bowel toxicity[84]. Tho and colleagues[81] evaluated the role of IMRT in 41 patients with locally advanced rectal cancer treated with preoperative 5FU CHRT. The results showed that IMRT provided dosimetric and radiobiological modeling benefits by reducing the dose to the small bowel, and the likelihood of late normal tissue complications. A dosimetric comparison of 3D-CRT using pelvic anatomical references, 3D-CRT with more restrictive volumes, and IMRT was explored by our institution in nine patients diagnosed with locally advanced rectal cancer. A number of parameters, such as conformity index in the planning target volume, different dose levels at the planning target volume and organs at risk were calculated and compared between the three plans. Target coverage was similar, but the conformity index was better using IMRT. Irradiation doses at small bowel and bladder were significantly reduced with IMRT planning.
Dosimetric parameters in rectal cancer with IMRT are encouraging. Clinical research looking for acute and late toxicity, tumor response, tumor control and survival is warranted. The rationale for the use of chemo-IMRT in locally advanced rectal cancer is based on the potential decrease of gastrointestinal toxicity while maintaining conventional dose to the primary tumor, draining lymph node regions and presacral region. This capacity to change the gastrointestinal toxicity profile may also allow reducing the number of fractions by increasing fraction size, which ultimately may improve the rate of pCR and cost-effectiveness.
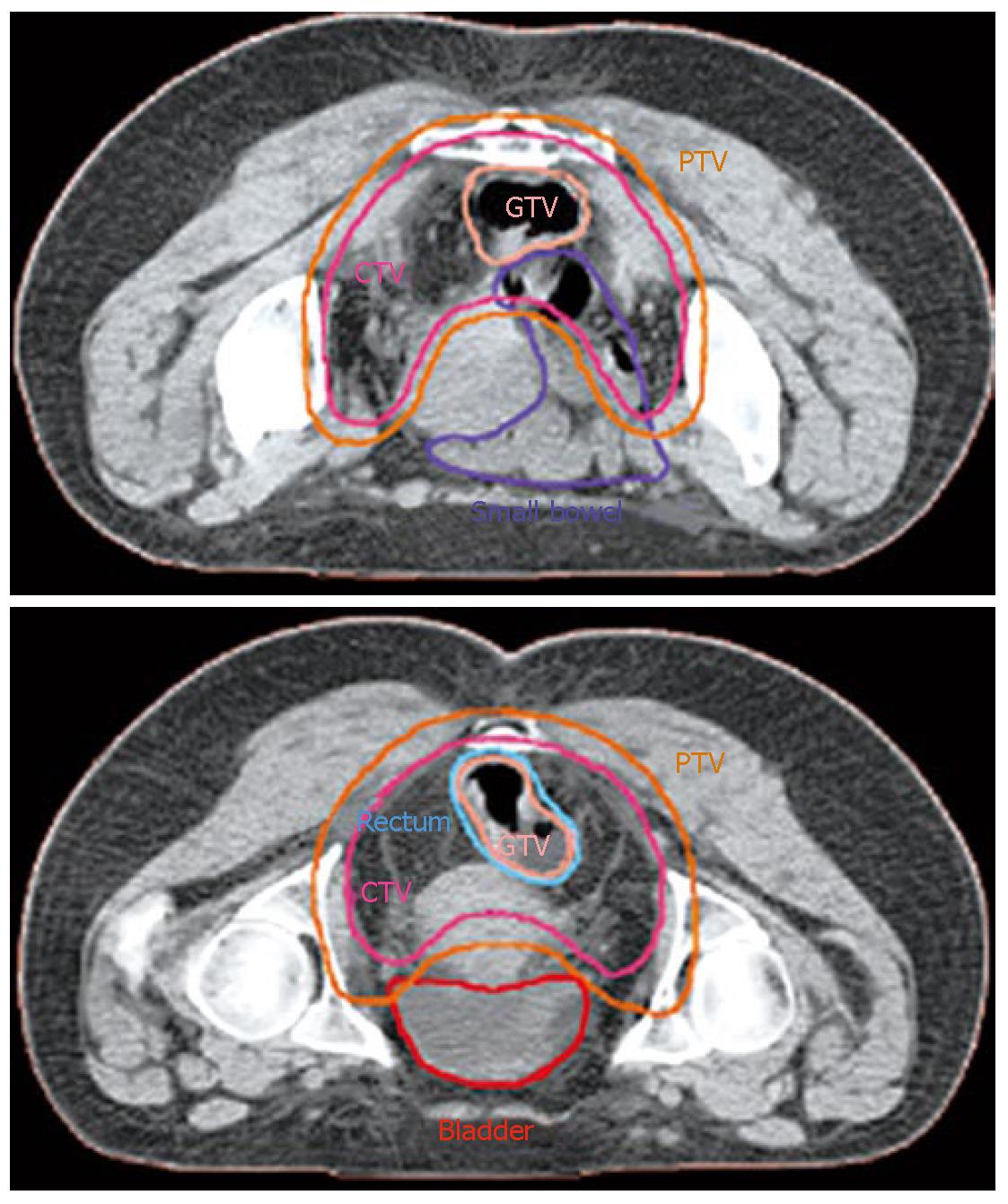
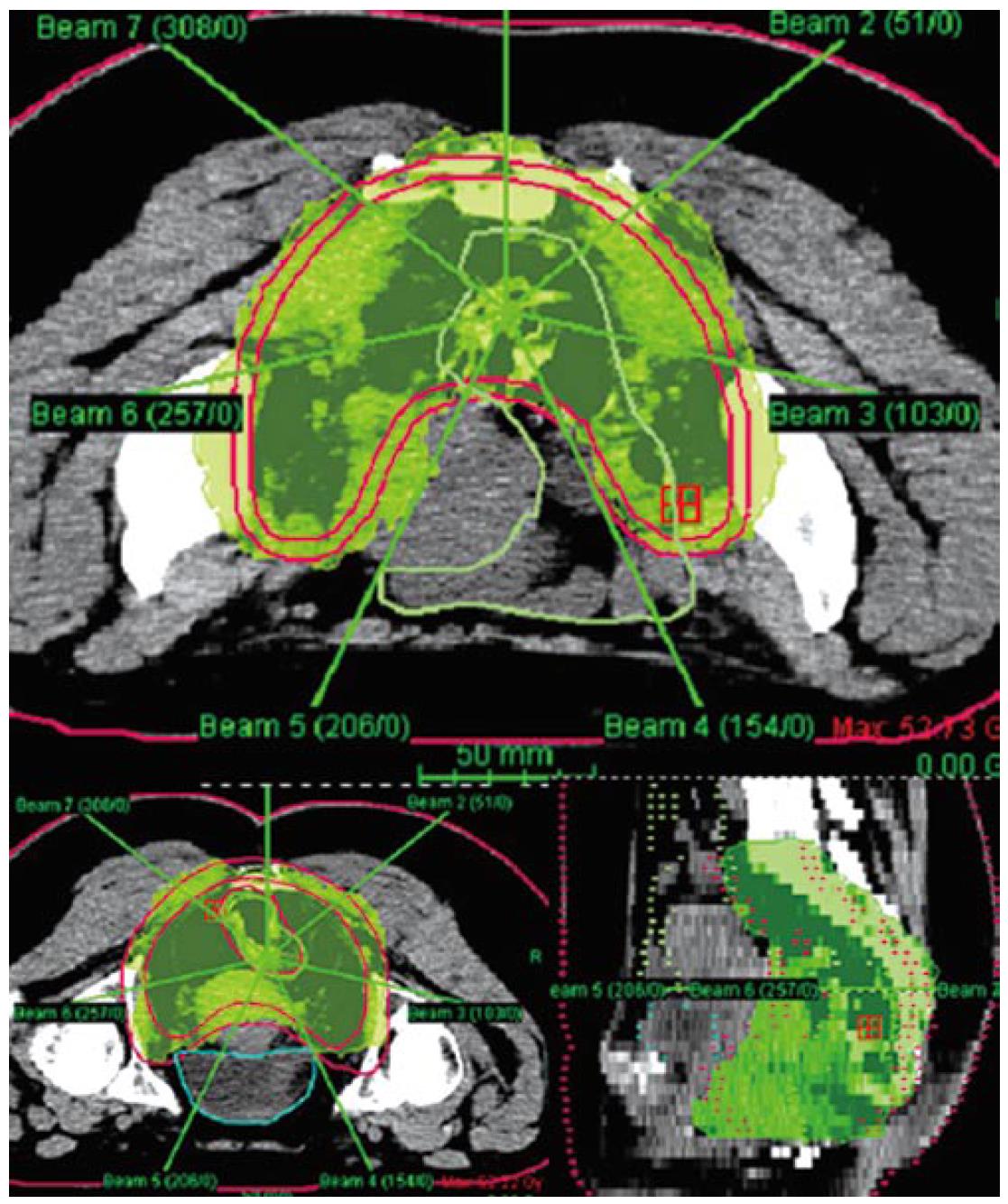
Our institution has carried out a prospective study of preoperative chemo-IMRT in rectal cancer. The treatment protocol includes simultaneous combination of capecitabine and oxaliplatin with three escalating dose levels of IMRT, 37.5 Gy 42.5 Gy and 47.5 Gy in 15, 17 and 19 fractions, respectively[85] Chemotherapy consisted on capecitabine 825 mg/m2 bid during radiation therapy (resting over the weekend) and oxaliplatin 60 mg/m2 d 1, 8 and 15. Resection was scheduled 6 wk after termination of chemo-IMRT. Simulation was made with the patient positioned prone and immobilized using a combination of prone head cushion and shell with a mixed foam bag. The patient was CT scanned from the L2 vertebral body to the entire perineum with a slice thickness of 5 mm. The slices were transferred through local network to the treatment planning system. The target volumes and organs at risk (OARs) were delineated on axial CT slices in the Helax-TMS treatment planning system (Nucletron Scandinavia, Uppsala, Sweden) as seen in Figure 1. The gross tumor volume (GTV) was defined as the primary tumor and the suspicious metastasic lymph nodes visualized on the CT scan. The clinical target volume (CTV) included the GTV, the presacral region and the common and internal iliac lymph nodes. Adding a margin of 0.5-1 cm around the CTV generated the planning target volume (PTV). The OARs outlined were the bladder and the small bowel. After the GTV, CTV, PTV and OARs were contoured the edited CT slices were transferred from the Helax-TMS treatment planning system to the inverse planning system (KonRad version 2, Siemens Oncology Care Systems, Heidelberg, Germany). Inverse planning for step-and-shoot treatment was performed using 15 MV photons generated on a Mevatron Primus linear accelerator (Siemens Oncology Care Systems, Concord, USA). Seven coplanar equally spaced fields (gantry angles 0°, 51°, 103°, 154°, 206°, 257° and 308°) were used and the isocenter was placed in the geometric center of the PTV. Figure 2 displays the clinical dosimetry over the patient CT scans.
The first three patients received 37.5 Gy and there were no dose-limiting toxicity (DLT) defined as any grade 3 or 4 gastrointestinal toxicities or grade 4 hematological toxicity. The next three patients received 42.5 Gy without observed DLT and the remaining patients received 47.5 Gy in 19 fractions. Preliminary data show that treatment compliance was 80%, grade 3 adverse events were seen in 21% of the cases, down staging was observed in 52% of patients and pathological response grade 3+ or 4 according to the scale established by Ruo et al[86] occurred in 45% of patients.
The use of preoperative IMRT combined with more active systemic chemotherapy provides a major challenge to improve treatment-related toxicity observed with more conventional radiation techniques. Furthermore, the promising favorable pathological response observed with these strategies has the potential to be associated with better loco regional control of disease and may predict better survival.
Preoperative CHRT followed by TME surgery is the current framework for rectal cancer treatment picture. Further advances with better agents (chemotherapy and molecular targeted therapies) and technology (IMRT) will be translated to improved shapes and colors, enhanced contrast and brightness: response intensity with balanced toxicity.
S- Editor Liu Y L- Editor Alpini GD E- Editor Ma WH
| 1. | Prolongation of the disease-free interval in surgically treated rectal carcinoma. Gastrointestinal Tumor Study Group. N Engl J Med. 1985;312:1465-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 913] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 2. | Douglass HO, Moertel CG, Mayer RJ, Thomas PR, Lindblad AS, Mittleman A, Stablein DM, Bruckner HW. Survival after postoperative combination treatment of rectal cancer. N Engl J Med. 1986;315:1294-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 357] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, Kubista TP, Poon MA, Meyers WC, Mailliard JA. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1419] [Cited by in RCA: 1303] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 4. | Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3104] [Cited by in RCA: 3114] [Article Influence: 129.8] [Reference Citation Analysis (0)] |
| 5. | Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med. 1997;336:980-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1815] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 6. | Cammà C, Giunta M, Fiorica F, Pagliaro L, Craxì A, Cottone M. Preoperative radiotherapy for resectable rectal cancer: A meta-analysis. JAMA. 2000;284:1008-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 551] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 7. | Bosset JF, Magnin V, Maingon P, Mantion G, Pelissier EP, Mercier M, Chaillard G, Horiot JC. Preoperative radiochemotherapy in rectal cancer: long-term results of a phase II trial. Int J Radiat Oncol Biol Phys. 2000;46:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 116] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Calvo FA, Gómez-Espí M, Díaz-González JA, Cantalapiedra R, Marcos P, Alvarado A, García Alfonso P, Herranz R, Alvarez E. Pathologic downstaging of T3-4Nx rectal cancer after chemoradiation: 5-fluorouracil vs. Tegafur. Int J Radiat Oncol Biol Phys. 2001;51:1264-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Chari RS, Tyler DS, Anscher MS, Russell L, Clary BM, Hathorn J, Seigler HF. Preoperative radiation and chemotherapy in the treatment of adenocarcinoma of the rectum. Ann Surg. 1995;221:778-786; discussion 786-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 191] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Guillem JG, Chessin DB, Cohen AM, Shia J, Mazumdar M, Enker W, Paty PB, Weiser MR, Klimstra D, Saltz L. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg. 2005;241:829-836; discussion 836-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 297] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 11. | Janjan NA, Khoo VS, Abbruzzese J, Pazdur R, Dubrow R, Cleary KR, Allen PK, Lynch PM, Glober G, Wolff R. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys. 1999;44:1027-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 351] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | Stryker SJ, Kiel KD, Rademaker A, Shaw JM, Ujiki GT, Poticha SM. Preoperative "chemoradiation" for stages II and III rectal carcinoma. Arch Surg. 1996;131:514-518; discussion 518-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Valentini V, Coco C, Cellini N, Picciocchi A, Fares MC, Rosetto ME, Mantini G, Morganti AG, Barbaro B, Cogliandolo S. Ten years of preoperative chemoradiation for extraperitoneal T3 rectal cancer: acute toxicity, tumor response, and sphincter preservation in three consecutive studies. Int J Radiat Oncol Biol Phys. 2001;51:371-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4455] [Article Influence: 212.1] [Reference Citation Analysis (1)] |
| 15. | Berger C, de Muret A, Garaud P, Chapet S, Bourlier P, Reynaud-Bougnoux A, Dorval E, de Calan L, Huten N, le Folch O. Preoperative radiotherapy (RT) for rectal cancer: predictive factors of tumor downstaging and residual tumor cell density (RTCD): prognostic implications. Int J Radiat Oncol Biol Phys. 1997;37:619-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 139] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, Souquet JC, Adeleine P, Gerard JP. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17:2396. [PubMed] |
| 17. | Valentini V, Coco C, Picciocchi A, Morganti AG, Trodella L, Ciabattoni A, Cellini F, Barbaro B, Cogliandolo S, Nuzzo G. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients. Int J Radiat Oncol Biol Phys. 2002;53:664-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 262] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | Crane CH, Skibber JM, Feig BW, Vauthey JN, Thames HD, Curley SA, Rodriguez-Bigas MA, Wolff RA, Ellis LM, Delclos ME. Response to preoperative chemoradiation increases the use of sphincter-preserving surgery in patients with locally advanced low rectal carcinoma. Cancer. 2003;97:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 144] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Crane CH, Skibber JM, Birnbaum EH, Feig BW, Singh AK, Delclos ME, Lin EH, Fleshman JW, Thames HD, Kodner IJ. The addition of continuous infusion 5-FU to preoperative radiation therapy increases tumor response, leading to increased sphincter preservation in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2003;57:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Grann A, Feng C, Wong D, Saltz L, Paty PP, Guillem JG, Cohen AM, Minsky BD. Preoperative combined modality therapy for clinically resectable uT3 rectal adenocarcinoma. Int J Radiat Oncol Biol Phys. 2001;49:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | García-Aguilar J, Hernandez de Anda E, Sirivongs P, Lee SH, Madoff RD, Rothenberger DA. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003;46:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 298] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 22. | O'Connell MJ, Martenson JA, Wieand HS, Krook JE, Macdonald JS, Haller DG, Mayer RJ, Gunderson LL, Rich TA. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 761] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 23. | Wolmark N, Wieand HS, Hyams DM, Colangelo L, Dimitrov NV, Romond EH, Wexler M, Prager D, Cruz AB, Gordon PH. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst. 2000;92:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 340] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 24. | Janjan NA, Abbruzzese J, Pazdur R, Khoo VS, Cleary K, Dubrow R, Ajani J, Rich TA, Goswitz MS, Evetts PA. Prognostic implications of response to preoperative infusional chemoradiation in locally advanced rectal cancer. Radiother Oncol. 1999;51:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 98] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Onaitis MW, Kirshbom PM, Hayward TZ, Quayle FJ, Feldman JM, Seigler HF, Tyler DS. Gastrointestinal carcinoids: characterization by site of origin and hormone production. Ann Surg. 2000;232:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Pucciarelli S, Friso ML, Toppan P, Fornasiero A, Carnio S, Marchiori E, Lise M. Preoperative combined radiotherapy and chemotherapy for middle and lower rectal cancer: preliminary results. Ann Surg Oncol. 2000;7:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Gunderson LL, Sargent DJ, Tepper JE, Wolmark N, O'Connell MJ, Begovic M, Allmer C, Colangelo L, Smalley SR, Haller DG. Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer: a pooled analysis. J Clin Oncol. 2004;22:1785-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 308] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 28. | Díaz-González JA, Calvo FA, Cortés J, García-Sabrido JL, Gómez-Espí M, Del Valle E, Muñoz-Jiménez F, Alvarez E. Prognostic factors for disease-free survival in patients with T3-4 or N+ rectal cancer treated with preoperative chemoradiation therapy, surgery, and intraoperative irradiation. Int J Radiat Oncol Biol Phys. 2006;64:1122-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Shia J, Guillem JG, Moore HG, Tickoo SK, Qin J, Ruo L, Suriawinata A, Paty PB, Minsky BD, Weiser MR. Patterns of morphologic alteration in residual rectal carcinoma following preoperative chemoradiation and their association with long-term outcome. Am J Surg Pathol. 2004;28:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Vecchio FM, Valentini V, Minsky BD, Padula GD, Venkatraman ES, Balducci M, Miccichè F, Ricci R, Morganti AG, Gambacorta MA. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys. 2005;62:752-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 329] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 31. | Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688-8696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 918] [Cited by in RCA: 950] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 32. | de la Torre A, Ramos S, Valcárcel FJ, Candal A, Regueiro CA, Romero J, Magallón R, Salinas J, de las Heras M, Veiras C. Phase II study of radiochemotherapy with UFT and low-dose oral leucovorin in patients with unresectable rectal cancer. Int J Radiat Oncol Biol Phys. 1999;45:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Díaz-González JA, Calvo FA, Cortés J, de La Mata D, Gómez-Espí M, Lozano MA, Lozano E, Serrano J, Herranz R. Preoperative chemoradiation with oral tegafur within a multidisciplinary therapeutic approach in patients with T3-4 rectal cancer. Int J Radiat Oncol Biol Phys. 2005;61:1378-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Feliu J, Calvilio J, Escribano A, de Castro J, Sánchez ME, Mata A, Espinosa E, García Grande A, Mateo A, González Barón M. Neoadjuvant therapy of rectal carcinoma with UFT-leucovorin plus radiotherapy. Ann Oncol. 2002;13:730-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Fernández-Martos C, Aparicio J, Bosch C, Torregrosa M, Campos JM, Garcera S, Vicent JM, Maestu I, Climent MA, Mengual JL. Preoperative uracil, tegafur, and concomitant radiotherapy in operable rectal cancer: a phase II multicenter study with 3 years' follow-Up. J Clin Oncol. 2004;22:3016-3022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Twelves C, Wong A, Nowacki MP, Abt M, Burris H, Carrato A, Cassidy J, Cervantes A, Fagerberg J, Georgoulias V. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 860] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 37. | Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, Shimma N, Umeda I, Ishitsuka H. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998;34:1274-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 929] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 38. | Sawada N, Ishikawa T, Sekiguchi F, Tanaka Y, Ishitsuka H. X-ray irradiation induces thymidine phosphorylase and enhances the efficacy of capecitabine (Xeloda) in human cancer xenografts. Clin Cancer Res. 1999;5:2948-2953. [PubMed] |
| 39. | Dunst J, Reese T, Sutter T, Zühlke H, Hinke A, Kölling-Schlebusch K, Frings S. Phase I trial evaluating the concurrent combination of radiotherapy and capecitabine in rectal cancer. J Clin Oncol. 2002;20:3983-3991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 163] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 40. | Ngan SY, Michael M, Mackay J, McKendrick J, Leong T, Lim Joon D, Zalcberg JR. A phase I trial of preoperative radiotherapy and capecitabine for locally advanced, potentially resectable rectal cancer. Br J Cancer. 2004;91:1019-1024. [PubMed] |
| 41. | De Paoli A, Chiara S, Luppi G, Friso ML, Beretta GD, Del Prete S, Pasetto L, Santantonio M, Sarti E, Mantello G. Capecitabine in combination with preoperative radiation therapy in locally advanced, resectable, rectal cancer: a multicentric phase II study. Ann Oncol. 2006;17:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Kim JS, Kim JS, Cho MJ, Song KS, Yoon WH. Preoperative chemoradiation using oral capecitabine in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2002;54:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 147] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 43. | Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2407] [Cited by in RCA: 2380] [Article Influence: 95.2] [Reference Citation Analysis (1)] |
| 44. | Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2273] [Cited by in RCA: 2221] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 45. | Minsky BD. Combined-modality therapy of rectal cancer with irinotecan-based regimens. Oncology (Williston Park). 2004;18:49-55. [PubMed] |
| 46. | Klautke G, Feyerherd P, Ludwig K, Prall F, Foitzik T, Fietkau R. Intensified concurrent chemoradiotherapy with 5-fluorouracil and irinotecan as neoadjuvant treatment in patients with locally advanced rectal cancer. Br J Cancer. 2005;92:1215-1220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Mehta VK, Cho C, Ford JM, Jambalos C, Poen J, Koong A, Lin A, Bastidas JA, Young H, Dunphy EP. Phase II trial of preoperative 3D conformal radiotherapy, protracted venous infusion 5-fluorouracil, and weekly CPT-11, followed by surgery for ultrasound-staged T3 rectal cancer. Int J Radiat Oncol Biol Phys. 2003;55:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 48. | Mohiuddin M, Winter K, Mitchell E, Hanna N, Yuen A, Nichols C, Shane R, Hayostek C, Willett C. Randomized phase II study of neoadjuvant combined-modality chemoradiation for distal rectal cancer: Radiation Therapy Oncology Group Trial 0012. J Clin Oncol. 2006;24:650-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 194] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 49. | Navarro M, Dotor E, Rivera F, Sánchez-Rovira P, Vega-Villegas ME, Cervantes A, García JL, Gallén M, Aranda E. A Phase II study of preoperative radiotherapy and concomitant weekly irinotecan in combination with protracted venous infusion 5-fluorouracil, for resectable locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Hofheinz RD, von Gerstenberg-Helldorf B, Wenz F, Gnad U, Kraus-Tiefenbacher U, Müldner A, Hehlmann R, Post S, Hochhaus A, Willeke F. Phase I trial of capecitabine and weekly irinotecan in combination with radiotherapy for neoadjuvant therapy of rectal cancer. J Clin Oncol. 2005;23:1350-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Klautke G, Küchenmeister U, Foitzik T, Ludwig K, Prall F, Klar E, Fietkau R. Concurrent chemoradiation with capecitabine and weekly irinotecan as preoperative treatment for rectal cancer: results from a phase I/II study. Br J Cancer. 2006;94:976-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2653] [Cited by in RCA: 2726] [Article Influence: 129.8] [Reference Citation Analysis (0)] |
| 53. | Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1720] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 54. | Kjellström J, Kjellén E, Johnsson A. In vitro radiosensitization by oxaliplatin and 5-fluorouracil in a human colon cancer cell line. Acta Oncol. 2005;44:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Aschele C, Friso ML, Pucciarelli S, Lonardi S, Sartor L, Fabris G, Urso ED, Del Bianco P, Sotti G, Lise M. A phase I-II study of weekly oxaliplatin, 5-fluorouracil continuous infusion and preoperative radiotherapy in locally advanced rectal cancer. Ann Oncol. 2005;16:1140-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 56. | Ryan DP, Niedzwiecki D, Hollis D, Mediema BE, Wadler S, Tepper JE, Goldberg RM, Mayer RJ. Phase I/II study of preoperative oxaliplatin, fluorouracil, and external-beam radiation therapy in patients with locally advanced rectal cancer: Cancer and Leukemia Group B 89901. J Clin Oncol. 2006;24:2557-2562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 57. | Turitto G, Panelli G, Frattolillo A, Auriemma A, de Luna FS, Cione G, De Angelis CP, Muto P, Iaffaioli RV. Phase II study of neoadjuvant concurrent chemioradiotherapy with oxaliplatin-containing regimen in locally advanced rectal cancer. Front Biosci. 2006;11:1275-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Borner MM, Dietrich D, Stupp R, Morant R, Honegger H, Wernli M, Herrmann R, Pestalozzi BC, Saletti P, Hanselmann S. Phase II study of capecitabine and oxaliplatin in first- and second-line treatment of advanced or metastatic colorectal cancer. J Clin Oncol. 2002;20:1759-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 144] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 59. | Díaz-Rubio E, Evans TR, Tabemero J, Cassidy J, Sastre J, Eatock M, Bisset D, Regueiro P, Baselga J. Capecitabine (Xeloda) in combination with oxaliplatin: a phase I, dose-escalation study in patients with advanced or metastatic solid tumors. Ann Oncol. 2002;13:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 60. | Cassidy J, Tabernero J, Twelves C, Brunet R, Butts C, Conroy T, Debraud F, Figer A, Grossmann J, Sawada N. XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol. 2004;22:2084-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 372] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 61. | Machiels JP, Duck L, Honhon B, Coster B, Coche JC, Scalliet P, Humblet Y, Aydin S, Kerger J, Remouchamps V. Phase II study of preoperative oxaliplatin, capecitabine and external beam radiotherapy in patients with rectal cancer: the RadiOxCape study. Ann Oncol. 2005;16:1898-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 62. | Rödel C, Grabenbauer GG, Papadopoulos T, Hohenberger W, Schmoll HJ, Sauer R. Phase I/II trial of capecitabine, oxaliplatin, and radiation for rectal cancer. J Clin Oncol. 2003;21:3098-3104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 217] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 63. | Glynne-Jones R, Sebag-Montefiore D, Maughan TS, Falk SJ, McDonald AC. A phase I dose escalation study of continuous oral capecitabine in combination with oxaliplatin and pelvic radiation (XELOX-RT) in patients with locally advanced rectal cancer. Ann Oncol. 2006;17:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 64. | Coleman CN. Linking radiation oncology and imaging through molecular biology (or now that therapy and diagnosis have separated, it's time to get together again!). Radiology. 2003;228:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 65. | Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2025] [Cited by in RCA: 2184] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 66. | Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1911] [Cited by in RCA: 1921] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 67. | Akimoto T, Hunter NR, Buchmiller L, Mason K, Ang KK, Milas L. Inverse relationship between epidermal growth factor receptor expression and radiocurability of murine carcinomas. Clin Cancer Res. 1999;5:2884-2890. [PubMed] |
| 68. | Liang K, Ang KK, Milas L, Hunter N, Fan Z. The epidermal growth factor receptor mediates radioresistance. Int J Radiat Oncol Biol Phys. 2003;57:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 214] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 69. | Wachsberger P, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: exploring mechanisms of interaction. Clin Cancer Res. 2003;9:1957-1971. [PubMed] |
| 70. | Sartor CI. Mechanisms of disease: Radiosensitization by epidermal growth factor receptor inhibitors. Nat Clin Pract Oncol. 2004;1:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 71. | Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4319] [Cited by in RCA: 3955] [Article Influence: 197.8] [Reference Citation Analysis (0)] |
| 72. | Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1468] [Cited by in RCA: 1441] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 73. | Willett CG, Boucher Y, Duda DG, di Tomaso E, Munn LL, Tong RT, Kozin SV, Petit L, Jain RK, Chung DC. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J Clin Oncol. 2005;23:8136-8139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 320] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 74. | Czito B, Bendell J, Willett C. Preliminary Results of a Phase I Study of External Beam Radiation Therapy (EBRT), Oxaliplatin (OX), Bevacizumab (BV), and Capecitabine (CAP) for Locally Advanced or Metastatic Adenocarcinoma of the Rectum. J Clin Oncol. 2006;24:157. |
| 75. | Thomas SM, Grandis JR. Pharmacokinetic and pharmacodynamic properties of EGFR inhibitors under clinical investigation. Cancer Treat Rev. 2004;30:255-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 76. | Saltz LB, Meropol NJ, Loehrer PJ, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1306] [Cited by in RCA: 1283] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 77. | Baumann M, Krause M. Targeting the epidermal growth factor receptor in radiotherapy: radiobiological mechanisms, preclinical and clinical results. Radiother Oncol. 2004;72:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 78. | Chung KY, Minsky BD, Schrag E. Phase I Trial of Preoperative Cetuximab With Concurrent Continuous Infusion 5-Fluorouracil and Pelvic Radiation in Patients With Local-Regionally Advanced Rectal Cancer. J Clin Oncol. 2006;24:161. |
| 79. | Machiels JP, Sempoux C, Scalliet P. Phase I Study of Preoperative Cetuximab, Capecitabine, and External Beam Radiotherapy in Patients With Rectal Cancer. J Clin Oncol. 2006;24:159. |
| 80. | Arnold D, Hipp R, Reese T. Phase I/II Study of Cetuximab, Capecitabine and Oxaliplatin (CAPOX) Combined With Standard Radiotherapy (RTX) As Neoadjuvant Treatment of Advanced Rectal Cancer (RC). J Clin Oncol. 2006;24:164. |
| 81. | Tho LM, Glegg M, Paterson J, Yap C, MacLeod A, McCabe M, McDonald AC. Acute small bowel toxicity and preoperative chemoradiotherapy for rectal cancer: investigating dose-volume relationships and role for inverse planning. Int J Radiat Oncol Biol Phys. 2006;66:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 82. | Baglan KL, Frazier RC, Yan D, Huang RR, Martinez AA, Robertson JM. The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2002;52:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 194] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 83. | Emami B. Medicine of IMRT. Intensity Modulated Radiation Therapy, a Clinical Perspective. Hamilton Ontario: BC Decker Inc 2005; 75-81. |
| 84. | Guerrero Urbano MT, Henrys AJ, Adams EJ, Norman AR, Bedford JL, Harrington KJ, Nutting CM, Dearnaley DP, Tait DM. Intensity-modulated radiotherapy in patients with locally advanced rectal cancer reduces volume of bowel treated to high dose levels. Int J Radiat Oncol Biol Phys. 2006;65:907-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 85. | Aristu J, Azcona JD, Moreno M, Martinez-Monge R. Rectal Cancer. Case Study. Intensity Modulated Radiation Therapy, a Clinical Perspective. Hamilton Ontario: BC Decker Inc 2005; 427-431. |
| 86. | Ruo L, Tickoo S, Klimstra DS, Minsky BD, Saltz L, Mazumdar M, Paty PB, Wong WD, Larson SM, Cohen AM. Long-term prognostic significance of extent of rectal cancer response to preoperative radiation and chemotherapy. Ann Surg. 2002;236:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 212] [Article Influence: 9.2] [Reference Citation Analysis (0)] |