Published online Nov 14, 2007. doi: 10.3748/wjg.v13.i42.5612
Revised: August 4, 2007
Accepted: August 31, 2007
Published online: November 14, 2007
AIM: To observe the apoptosis and oncosis of pancreatic acinar cells and secondary inflammatory reaction in pancreatic tissue from rats with acute pancreatitis (AP), and the influences of artemisinin on them.
METHODS: AP was induced by 4 intraperitoneal injections of caerulein at 1 h intervals. To induce apoptosis, solution of artemisinin (50 mg/kg) was given intraperitoneally 1, 12, 24 and 36 h after the last caerulein injection. Histological examination of impairment of pancreatic tissue and detection of serum amylase were performed to evaluate the severity of acute pancreatitis. Apoptosis and oncosis were detected with acridine orange (AO) and ethylene dibromide (EB) staining. Caspase-3 and myeloperoxidase (MPO) activity were measured by colorimetric assay. Nuclear factor-kappa B (NF-κB) activation was detected by flow cytometry. Macrophage inflammatory protein-1α (MIP-1α) protein was measured by Western blot. Interleukin-1β (IL-1β) mRNA was detected by RT-PCR.
RESULTS: Addition of artemisinin increased the number of apoptotic cells (11.7% ± 1.4% vs 6.3% ± 0.7%, P < 0.05), while reduced the number of oncotic cells (13.0% ± 2.4% vs 17.5% ± 2.2%, P < 0.05). The activity of caspase-3 speeded up (1.52 ± 0.21 vs 1.03 ± 0.08, P < 0.05), the pancreas pathological impairment was relieved (3.0 ± 0.5 vs 4.0 ± 0.5, P < 0.05) and the level of serum amylase decreased (5642 ± 721 U/dL vs 7821 ± 653 U/dL, P < 0.05). The activation of NF-κB (29% ± 4.1% vs 42% ± 5.8%), MIP-1α protein (3.7 ± 0.5 vs 5.8 ± 0.7), MPO (0.52 ± 0.06 U/g vs 0.68 ± 0.09 U/g), IL-1β mRNA (1.7 ± 0.3 vs 2.4 ± 0.4) in the apoptosis inducing group was obviously decreased (P < 0.05).
CONCLUSION: Inducing apoptosis can relieve pathological impairment and inflammatory reaction in AP rats.
- Citation: Zhao M, Xue DB, Zheng B, Zhang WH, Pan SH, Sun B. Induction of apoptosis by artemisinin relieving the severity of inflammation in caerulein-induced acute pancreatitis. World J Gastroenterol 2007; 13(42): 5612-5617
- URL: https://www.wjgnet.com/1007-9327/full/v13/i42/5612.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i42.5612
Many factors lead to acute pancreatitis (AP). A series of cascade reactions of inflammatory mediators and over-activation of leukocytes are the important causes for systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS)[1]. Although anti-cytokine therapy is able to relieve the severity of AP, it is difficult to block each pathway due to the complicated network of cytokines[2]. Therefore, knowing how to inhibit the initial inflammatory reaction is the key to the treatment of AP. It was reported that inflammatory reaction is correlated to the death modes of pancreatic acinar cells[3]. If death of pancreatic acinar cells occurs in the mode of apoptosis, the cell membrane is intact and there is no release of inflammatory mediators and pancreatin, the inflammatory reaction may be mild[4]. However, if death of pancreatic acinar cells occurs in the mode of oncosis, various pancreatin and inflammatory mediators may release, thus causing a variety of inflammatory cell aggregations and inducing intense inflammatory reactions[5]. If we can induce apoptosis and reduce oncosis, intense inflammatory reactions may be inhibited.
In this study, apoptosis of pancreatic acinar cells was induced by the apoptosis inductor-artemisinin. Changes in apoptosis, oncosis and secondary inflammatory reaction were observed.
Twenty-four male Wistar rats (200 g ± 20 g) were provided by the Animal Research Center of the First Clinical College of Harbin Medical University (Harbin, China), and divided into 3 groups (8 rats in each group): control group, AP group, and artemisinin-treated group (apoptosis inducing group). AP was induced by 4 intraperitoneal injections of caerulein (20 μg/kg, Sigma, USA) at 1 h intervals. To induce apoptosis, solution of artemisinin (2 mg/kg, Huaxin, Sichuan, China) was given intraperitoneally 1, 12, 24 and 36 h after the last caerulein injection. The control rats were only given saline solution. Forty-eight hours after the final injection of caerulein, rats were anaesthetized with sodium pentobarbital (40 mg/kg), and then a laparotomy was performed with the pancreas rapidly removed for further analyses.
Samples of pancreatic tissue were fixed in 20% formal-dehyde and processed for paraffin histology. After staining with hematoxylin and eosin (HE), histological grading of interlobular edema, inflammatory infiltration, parenchyma hemorrhage, parenchyma necrosis and vacuolization was valued as previously described[6], then a pathologic score was calculated based on these light microscopic examinations.
Serum was collected from the rats for amylase measurement. Amylase level was determined using a commercial chro-matometric kit (Jiancheng, Nanjing, China).
Pancreatic acinar cells were isolated from Wistar rats by two-step collagenase digestion[7] and loaded onto slides with AO (10 μg/mL, Sigma, USA) and EB (10 μg/mL, Sigma, USA) for 10 min. The slides were scanned under confocal laser microscope (Zeiss, Germany) and 500 cells were counted under fluorescent microscope (Nikon, Japan).
Caspase-3 activity was measured using a colorimetric assay kit (KeyGEN, Nanjing, China) according to the manufacturer's instructions. After isolation by two-step collagenase digestion, pancreatic acinar cells were mixed with 50 μL lysis buffer, the supernatant was mixed with 5 μL caspase substrate and 50 μL reaction buffer, and incubated at 37°C for 4 h in the dark. Fluorescence intensity of the caspase substrate was measured photometrically at 405 nm.
Fresh pancreatic tissues were sheared into pieces of 1.0 mm3 with scissors, and centrifuged at 200 ×g for 5 min. Then 10 mL detergent solution containing 1% TritonX-100 (Sigma,USA) was added, and stored in a refrigerator at 4°C for 18 h, then filtered through 50 μm nylon meshes. For fluorescent labeling, 1 μL RNAase was added into 50 μL nucleus suspension and water-bath at 37°C for 30 min, then 40 μL NF-κB p65 monoclonal antibody (Santa Cruz, USA) was added and incubated at room temperature for 20 min. The samples were treated with 1 μL FITC-labeled second antibody (Jackson Immuno Research, USA) and incubated at room temperature for another 20 min. After treated with 20 μL propidium iodide (PI, Sigma, USA) for 30 min in a dark room, the samples were analyzed using a FACScan flow cytometer (Becton Dicknson, USA).
MIP-1α was detected by Western blot analysis, total protein extract was separated by 10% SDS/PAGE before it was transferred electrophoretically (100 V, 1 h) to hybond C membrane. The membranes were probed with 10 μL anti-MIP-1α monoclonal antibody (Santa Cruz, USA) in TBS-T (1:500), and the immunocomplexed membranes were re-probed at room temperature for 1 h with horseradish peroxidase-conjugated anti-rabbit secondary antibody (Jackson Immuno Research, USA) in TBS-T (1:500), with 5% blocking reagent. At last, the immunoreactive proteins were visualized using the ECL Western blot analysis system (Amersham, UK).
The pancreatic tissue was frozen in liquid nitrogen and homogenated. MPO activity was detected with a chromatometric kit (Jianchen, Nanjing, China) following the manufacturer's instructions, and data were expressed as the change in absorbance at 460 nm.
Total RNA was extracted from pancreatic tissue using Trizol reagent (Invitrogen, USA) and reversely transcriped into cDNA according to the instructions of the kit (Promega, USA). The resulting cDNA was used as a template for subsequent polymerase chain reaction (PCR). The sequences of rat-specific primers for IL-1β (519 bp) are as follows: 5'CCAGGATGAGGACCCAAGCA3' (sense), 5'TCCCGACCATTGCTGTTTCC3' (antisense). Housekeeping gene β-actin (348 bp) was used as a controller, 5'CATCACCATTGGCAATGAGCG3' (sense), 5'CTAGAAGCATTTGCGGTCGGAC3' (antisense). The PCR products were resolved in 1.0% agarose gel for electrophoresis and photographed under ultraviolet transillumination and the intensity of PCR products was measured using a video image analysis system.
Data were expressed as mean ± SD. Differences between the groups were tested for significance by one-way analysis of variance (ANOVA), and intergroup comparison was made by Student-Newman-Keuls test. P < 0.05 was considered statistically significant.
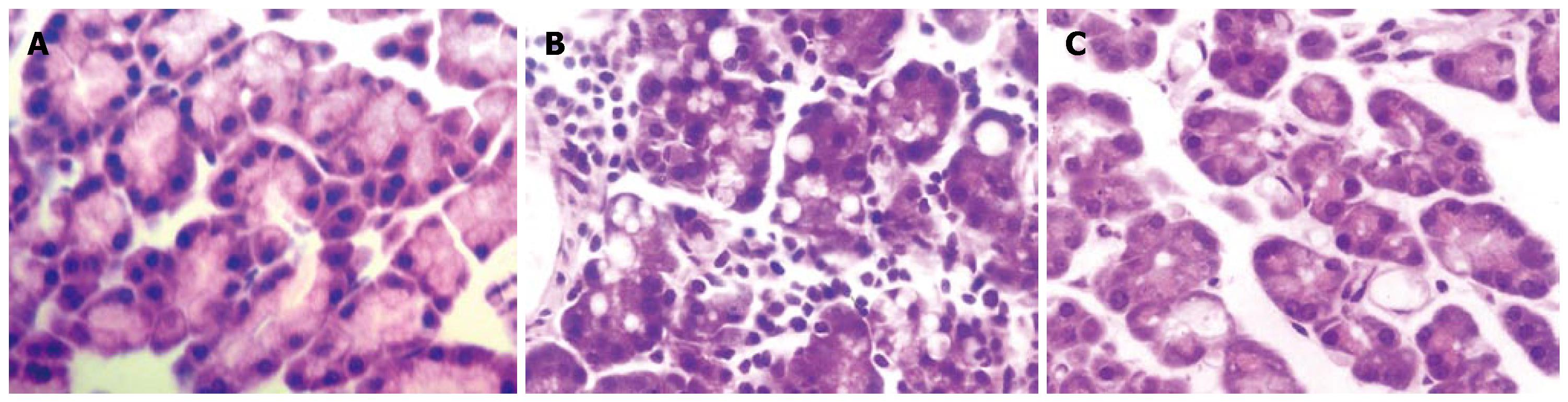
Pancreatic tissue was normal in the control group with a low pathological score. In the AP group, pancreatic tissue displayed lobular mesenchymal rarefaction, edema and inflammatory cell infiltration. In contrast, in the artemisinin-treated group, edema and inflammatory cell infiltration were significantly relieved compared with the AP group (Figure 1). The pathological score showed alleviated pathological impairment in pancreatic tissue after treated with artemisinin (P < 0.05 vs the AP group) (Table 1).
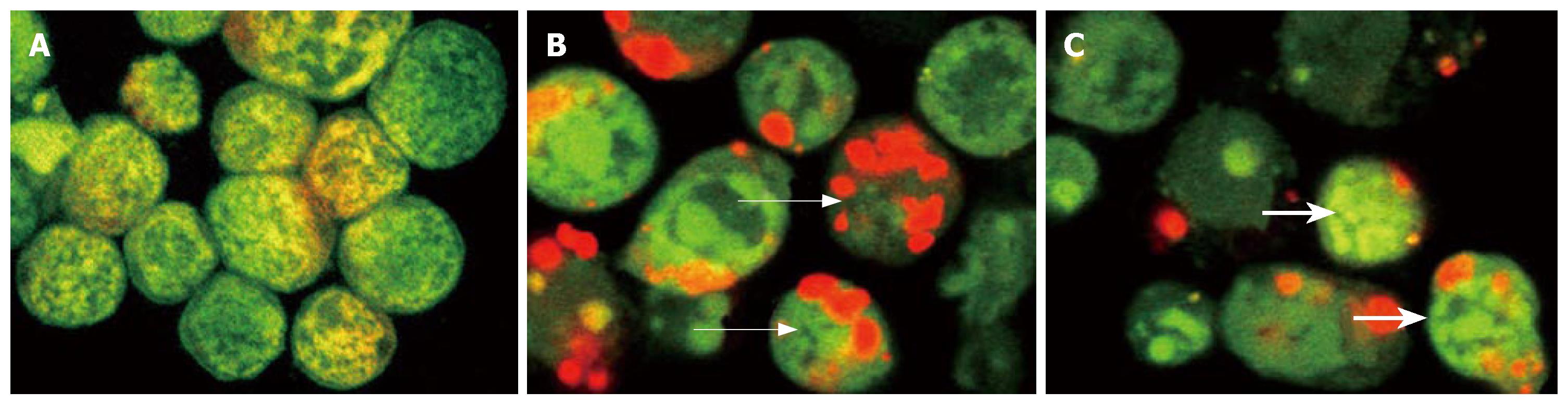
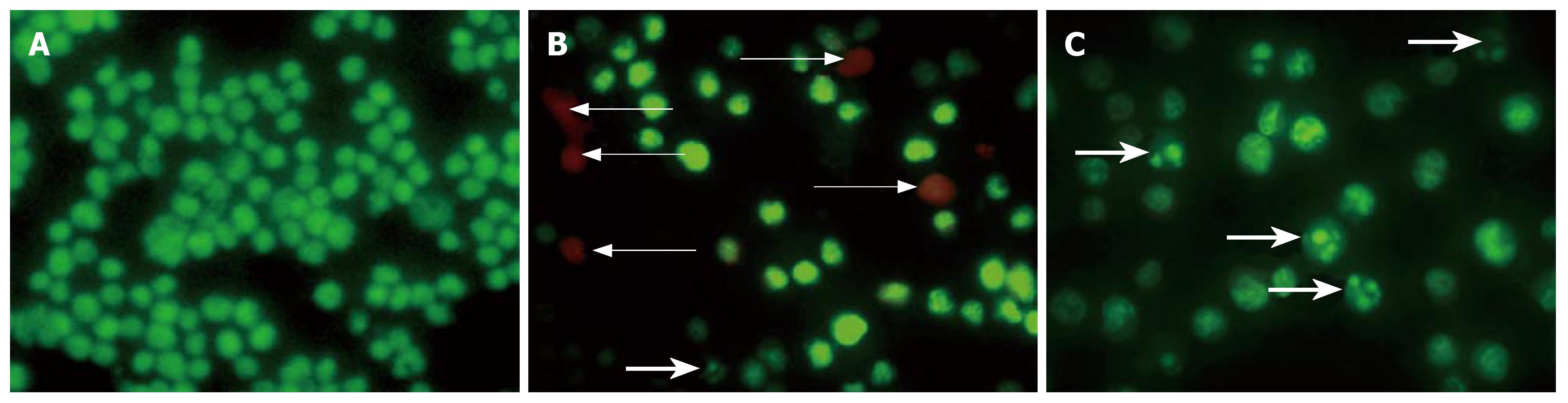
As shown in Figure 2, the nuclei of normal cells showed normal morphology of green fluorescence, while apoptotic cells showed shrunk, condensed or splitted nuclei (green). EB could be resisted by the intact cytoplasmic membrane of normal and apoptotic cells. EB could penetrate the cytoplasmic membrane of oncotic cells, and stain the nuclei of orange-stained cells. So, the apoptotic index or oncotic index, i.e., the number of apoptotic cells or oncotic cells per 100 cells, could be calculated (Figure 3). The results indicate that only sporadic apoptotic or oncotic cells were observed in the control group, but more in the AP and artemisinin-treated groups. In the AP group, there were less apoptotic cells and more oncotic cells. The number of apoptotic cells increased and the number of oncotic cells decreased significantly in the artemisinin-treated group (P < 0.05) (Table 1).
The activity of caspase-3 in isolated pancreatic acinar cells was low in the control group, and high in the AP group, which was significantly elevated after apoptosis was induced by artemisinin (P < 0.05) (Table 1).
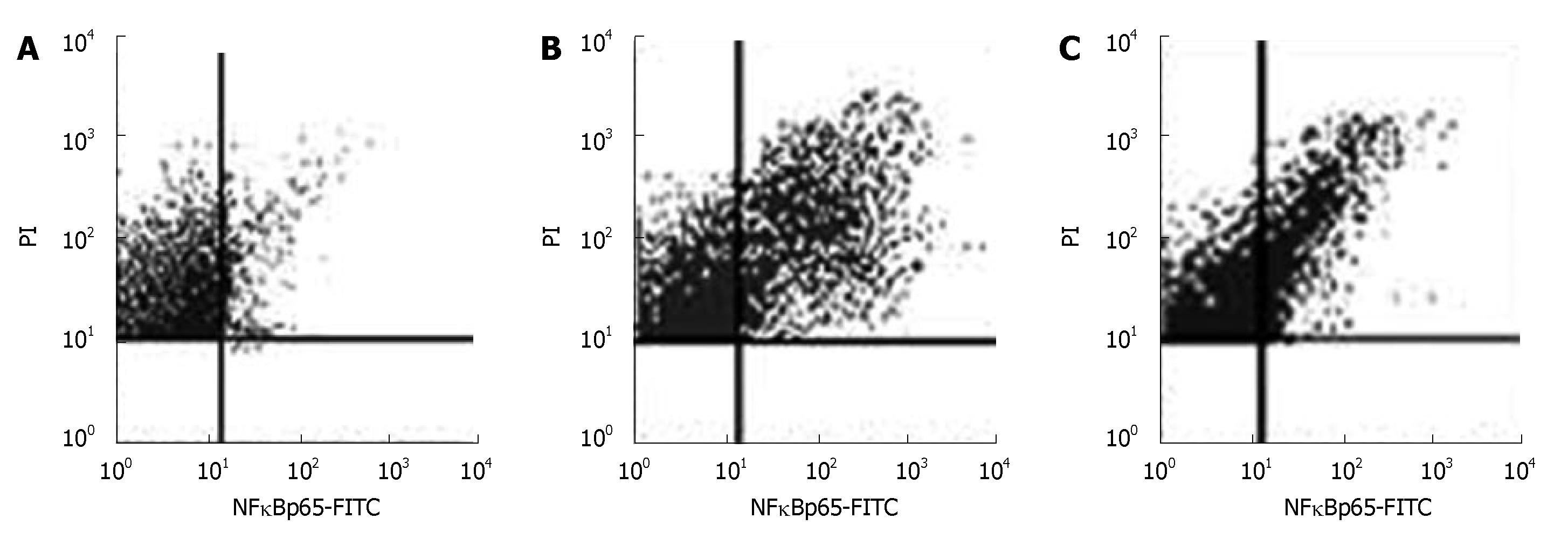
Activation of NF-κB in normal pancreatic nuclei was significantly higher in the AP group than in the control group (P < 0.05) (Figure 4, Table 2).
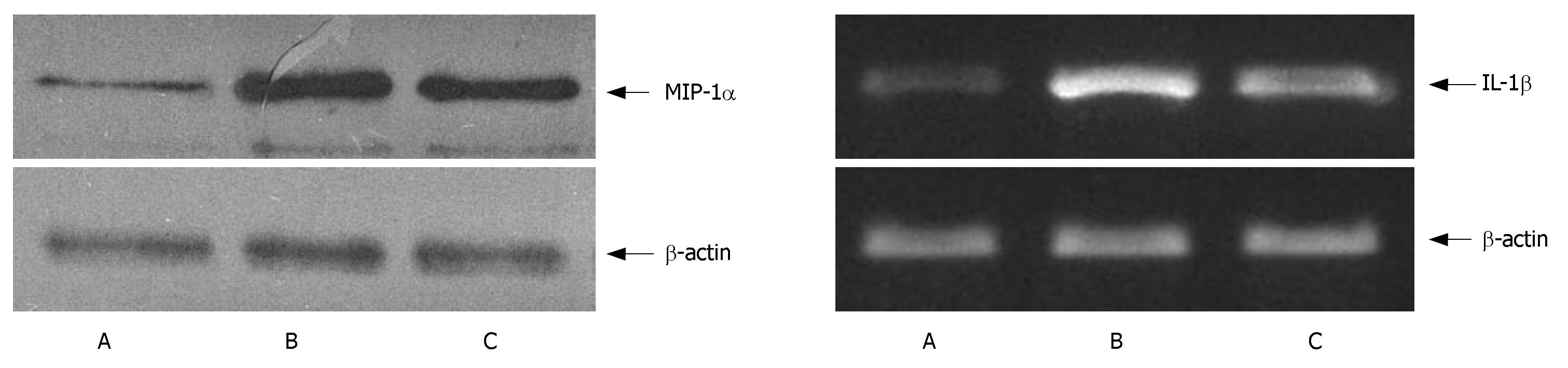
MIP-1α level was low in the control group and high in the AP group. It was obviously lower in the artemisinin-treated group than in the AP group (P < 0.05) (Figure 5, Table 2).
The expression of IL-1β mRNA was low in the control group and high in the AP group. It was lower in the artemisinin-treated group than in the AP group (P < 0.05) (Figure 5, Table 2).
Since each neutrophil granulocyte contains a certain quantity of MPO, detection of MPO in pancreatic tissue reflects the degree of neutrophil infiltration of the tissue. In our study, the MPO concentration was low in the control group and high in the AP group. However, it was down-regulated after induction of apoptosis (P < 0.05) (Table 2).
AP characterized not only by pancreas impairment, but also by inflammatory cell infiltration and release of various kinds of inflammatory mediators, can develop into SIRS and MODS in some cases and endanger their life[8,9]. Initiation of inflammatory reaction is related to the death pathway of impaired pancreatic acinar cells[10]. Since the conception of apoptosis was proposed by Kerr in 1972[11], apoptosis has been extensively studied. In recent years, attention has been paid to another cell death pathway-oncosis, and it was gradually realized that oncosis makes no less sense than apoptosis[12]. Oncosis has a feature of cell swelling, and cell membrane integrity is destroyed and DNA is split into non-specific fragments. Finally the cells are dissolved accompanying inflammatory reaction. In some physiological and pathological processes, these two kinds of death pathways exist simultaneously, and may interconvert to each other under certain conditions[13]. Oncotic cells may release much more entocytes (including digestive enzyme and inflammatory active medium). It not only destroys the local tissue, but also activates mononuclear cells, leading to SIRS. However, apoptosis may not cause secondary inflammation. During AP, the patient has his or her own self-regulating mechanism. Under certain conditions, the apoptotic signal conduction pathway can be initiated, and more apoptosis will be induced and harmful effects of oncosis may be relieved. However, if AP develops quickly or the patients' self-regulation is in disorder, the apoptosis signal conduction pathway cannot be initiated in time, causing predominant oncosis, leading to aggravation of the disease. This has been proved by Kaiser et al[14], who found that when apoptosis is inhibited by cycloheximide, AP obviously aggravates. However, Bhatia et al[15] induced apoptosis during their experiment, resulting in the relief of AP. It was reported that one of the therapeutic mechanisms of somatostatin analogue, the most effective drug for AP, is to induce apoptosis of impaired pancreatic acinar cells[16]. Whether AP can be controlled by inducing apoptosis of pancreatic acinar cells has been extensively studied.
Artemisinin is an important active component of tra-ditional Chinese medicine which can induce apoptosis[17,18]. Hahm et al[19] found that apoptotic index is elevated but pathological changes in AP rats treated with DA-9610 (an extract from Artemisia Asiatica). In this study, artemisinin was used as an apoptosis inductor. After artemisinin was added into pancreatic acinar cells stimulated by caerulein, apoptosis and oncosis were detected with AO and EB staining, the number of apoptotic cells increased, but the number of oncotic cells decreased. Caspase-3 is a key molecule in the process of apoptosis. Generally, the precursor of caspase-3 in cells is incompetent. However, if it is activated, it can cleave important structural and functional proteins inside the cells and cause chromosome condensation, DNA fragmentation, nuclear membrane rupture, etc, finally resulting in apoptosis. The presence of activated caspase-3 indicates that apoptosis is at the irreversible stage[20]. Nam et al[21] found that artemisinin can induce apoptosis by up-regulating caspase-3. Our study also showed that the activity of caspase-3 in pancreatic acinar cells was obviously elevated after artemisinin was added, indicating that artemisinin may promote apoptosis. We examined the pancreatic tissue with HE staining and found that after induction of apoptosis, infiltration of inflammatory cells decreased and the pancreas impairment was relieved. Based on this understanding, we observed the degree of inflammatory reaction after induction of apoptosis.
We detected transcription factor-NF-κB which regulates synthesis of many inflammatory mediators and cytokines[22]. NF-κB is a protein that regulates gene transcription, participates in regulating many inflammatory factors, and evokes immune and inflammatory reactions[23]. NF-κB plays a key role in the development of AP[24]. In our study, activation of NF-κB in the apoptosis inducing group was obviously decreased, compared with the AP group, indicating that activation of NF-κB can be decreased by inducing apoptosis and reducing oncosis. The conserved sequence of MIP-1α combined with NF-κB exists in its promoter region[25], suggests that MIP-1α may be one of the downstream targets regulated by NF-κB. MIP-1α was detected by Western blot assay in this study, proving that if apoptosis is induced, MIP-1α can be inhibited. MIP-1α is a CC-type chemotatic factor and plays an important role in recruiting mononuclear cells and lymphocytes[26]. Just as the "over-activation of leucocyte theory" proposed by Rindernech et al[27], AP aggravates because inflammatory cells are over-activated, and these activated cells such as granulocytes and macrophages, play a great role in the development of AP. Therefore, MPO (the marker of neutrophils) and IL-1β (the inflammatory factors generated mainly by mononuclear macrophages)[28] were detected in this study, indicating that MPO is obviously decreased in pancreas tissue after induction of apoptosis, reducing neutrophil recruition and infiltration to pancreas tissue. IL-1β is a kind of proinflammatory cytokines mainly generated by macrophages in pancreas when AP occurs. IL-1β can activate neutrophils, up-regulate the expression of surface adhesion molecules of lymphocyte and endotheliocytes[29]. Fink et al[30] reported that release of pancreatic amylase and necrosis of pancreas tissue are obviously decreased by blocking IL-1 receptor, demonstrating that IL-1 is essential to inflammation and development of AP. Our study proved that after induction of apoptosis, the level of IL-1β mRNA in pancreas tissue was low, indicating that infiltration and activation of macrophages are decreased and inflammatory reaction is inhibited after induction of apoptosis.
In conclusion, infiltration of inflammatory cells and generation of inflammatory cytokines can be decreased by inducing apoptosis and reducing oncosis of pancreatic acinar cells.
One of the greatest findings in AP is the initiation of cytokine network in AP patients that promotes occurrence of SIRS and MODS. Efforts have been made to alleviate pathological changes in AP by inhibiting the cytokine network. Since cytokine network is so complex that it is impossible to block all the pathways, it may pave a new way for the treatment of AP to obstruct the cytokine chain reactions. It was reported that the cytokine network can be obstructed by inducing apoptosis, decreasing oncosis and release of endocellular enzyme, suggesting that any drugs regulating apoptosis may be used in the treatment of AP.
It has been verified that AP worsens when apoptosis of pancreatic acinar cells is inhibited by cycloheximide, and that AP is alleviated when apoptosis is induced. It was reported that the severity of AP is associated with the degree of oncosis. Hahm et al found that there is an elevated apoptotic index but extenuated pathological changes in AP rats after treated with DA-9610 (an extract from Artemisia Asiatica), suggesting that artemisinin can regulate cell death and can be used in the treatment of AP.
When the concept of apoptosis was first put forward by Kerr in 1972, a lot of studies have been performed with it. In recent years, more and more attention has been paid to oncosis, showing that the importance of oncosis is no less than that of apoptosis. In traditional Chinese medicine, some important prescriptions have been successfully applied in AP treatment, and one of the mechanisms is to suppress inflammatory response and induce apoptosis. Artemisinin is an important active component of traditional Chinese medicine which can induce apoptosis. In this study, we observed the regulatory effect of artemisinin on apoptosis and oncosis of pancreatic acinar cells and its therapeutic effect on AP.
Artemisinin can induce apoptosis and reduce oncosis of pancreatic acinar cells at the onset of AP, thus repressing the intense inflammatory reactions, such as SIRS and MODS. Therefore, there is a bright prospect for artemisinin in the treatment of AP.
Oncosis, or cellular swelling, is a pathological process of cell death, in which the completeness of cell membrane is destructed and DNA is split to non-specific fragments, ultimately leading to cell lysis complicated by inflammatory reactions.
The effect of artemisinin on acute pancreatitis was analyzed. The data presented are interesting.
S- Editor Zhu LH L- Editor Wang XL E- Editor Liu Y
| 1. | Chan YC, Leung PS. Acute pancreatitis: animal models and recent advances in basic research. Pancreas. 2007;34:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Zhang XP, Lin Q, Zhou YF. Progress of study on the relationship between mediators of inflammation and apoptosis in acute pancreatitis. Dig Dis Sci. 2007;52:1199-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Malka D, Vasseur S, Bödeker H, Ortiz EM, Dusetti NJ, Verrando P, Dagorn JC, Iovanna JL. Tumor necrosis factor alpha triggers antiapoptotic mechanisms in rat pancreatic cells through pancreatitis-associated protein I activation. Gastroenterology. 2000;119:816-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | McHugh P, Turina M. Apoptosis and necrosis: a review for surgeons. Surg Infect (Larchmt). 2006;7:53-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Criddle DN, Gerasimenko JV, Baumgartner HK, Jaffar M, Voronina S, Sutton R, Petersen OH, Gerasimenko OV. Calcium signalling and pancreatic cell death: apoptosis or necrosis? Cell Death Differ. 2007;14:1285-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Grewal HP, Mohey el Din A, Gaber L, Kotb M, Gaber AO. Amelioration of the physiologic and biochemical changes of acute pancreatitis using an anti-TNF-alpha polyclonal antibody. Am J Surg. 1994;167:214-218; discussion 218-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 142] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Zhang H, Li YY, Wang SN, Zhang KH, Wu XZ. Effects of lipopolysaccharides on calcium homeostasis in isolated pancreatic acinar cells of rat. Acta Pharmacol Sin. 2003;24:790-795. [PubMed] |
| 8. | Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, Chevali L. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 388] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 9. | Cuzzocrea S, Nocentini G, Di Paola R, Agostini M, Mazzon E, Ronchetti S, Crisafulli C, Esposito E, Caputi AP, Riccardi C. Proinflammatory role of glucocorticoid-induced TNF receptor-related gene in acute lung inflammation. J Immunol. 2006;177:631-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Andersson R, Wang X. Patterns of pancreatic cell death: apoptosis versus oncosis. Pancreas. 1998;17:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Schattenberg JM, Galle PR, Schuchmann M. Apoptosis in liver disease. Liver Int. 2006;26:904-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Jugdutt BI, Idikio HA. Apoptosis and oncosis in acute coronary syndromes: assessment and implications. Mol Cell Biochem. 2005;270:177-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Van Cruchten S, Van Den Broeck W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol. 2002;31:214-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 343] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 14. | Kaiser AM, Saluja AK, Lu L, Yamanaka K, Yamaguchi Y, Steer ML. Effects of cycloheximide on pancreatic endonuclease activity, apoptosis, and severity of acute pancreatitis. Am J Physiol. 1996;271:C982-C993. [PubMed] |
| 15. | Bhatia M, Wallig MA, Hofbauer B, Lee HS, Frossard JL, Steer ML, Saluja AK. Induction of apoptosis in pancreatic acinar cells reduces the severity of acute pancreatitis. Biochem Biophys Res Commun. 1998;246:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 101] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Yuan Y, Gong Z, Lou K, Tu S, Di Z, Xu J. Effects and mechanisms of somatostatin analogs on apoptosis of pancreatic acinar cells in acute pancreatitis in mice. J Gastroenterol Hepatol. 2001;16:683-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Mercer AE, Maggs JL, Sun XM, Cohen GM, Chadwick J, O'Neill PM, Park BK. Evidence for the involvement of carbon-centered radicals in the induction of apoptotic cell death by artemisinin compounds. J Biol Chem. 2007;282:9372-9382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Efferth T. Molecular pharmacology and pharmacogenomics of artemisinin and its derivatives in cancer cells. Curr Drug Targets. 2006;7:407-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Hahm KB, Kim JH, You BM, Kim YS, Cho SW, Yim H, Ahn BO, Kim WB. Induction of apoptosis with an extract of Artemisia asiatica attenuates the severity of cerulein-induced pancreatitis in rats. Pancreas. 1998;17:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Yasuda T, Takeyama Y, Ueda T, Shinzeki M, Kishi S, Sawa H, Nakajima T, Kuroda Y. Protective effect of caspase inhibitor on intestinal integrity in experimental severe acute pancreatitis. J Surg Res. 2007;138:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Nam W, Tak J, Ryu JK, Jung M, Yook JI, Kim HJ, Cha IH. Effects of artemisinin and its derivatives on growth inhibition and apoptosis of oral cancer cells. Head Neck. 2007;29:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Radisky DC, Bissell MJ. NF-kappaB links oestrogen receptor signalling and EMT. Nat Cell Biol. 2007;9:361-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Shi C, Zhao X, Wang X, Andersson R. Role of nuclear factor-kappaB, reactive oxygen species and cellular signaling in the early phase of acute pancreatitis. Scand J Gastroenterol. 2005;40:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Folch-Puy E, Granell S, Iovanna JL, Barthet M, Closa D. Peroxisome proliferator-activated receptor gamma agonist reduces the severity of post-ERCP pancreatitis in rats. World J Gastroenterol. 2006;12:6458-6463. [PubMed] |
| 25. | Monaco C, Andreakos E, Kiriakidis S, Mauri C, Bicknell C, Foxwell B, Cheshire N, Paleolog E, Feldmann M. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci USA. 2004;101:5634-5639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 276] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 26. | Brueckmann M, Hoffmann U, Dvortsak E, Lang S, Kaden JJ, Borggrefe M, Haase KK. Drotrecogin alfa (activated) inhibits NF-kappa B activation and MIP-1-alpha release from isolated mononuclear cells of patients with severe sepsis. Inflamm Res. 2004;53:528-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Rinderknecht H. Fatal pancreatitis, a consequence of excessive leukocyte stimulation? Int J Pancreatol. 1988;3:105-112. [PubMed] |
| 28. | Fairweather D, Frisancho-Kiss S, Njoku DB, Nyland JF, Kaya Z, Yusung SA, Davis SE, Frisancho JA, Barrett MA, Rose NR. Complement receptor 1 and 2 deficiency increases coxsackievirus B3-induced myocarditis, dilated cardiomyopathy, and heart failure by increasing macrophages, IL-1beta, and immune complex deposition in the heart. J Immunol. 2006;176:3516-3524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | van Griensven M, Probst C, Müller K, Hoevel P, Pape HC. Leukocyte-endothelial interactions via ICAM-1 are detrimental in polymicrobial sepsis. Shock. 2006;25:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Fink G, Yang J, Carter G, Norman J. Acute pancreatitis-induced enzyme release and necrosis are attenuated by IL-1 antagonism through an indirect mechanism. J Surg Res. 1997;67:94-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |