Published online Nov 14, 2007. doi: 10.3748/wjg.v13.i42.5547
Revised: August 10, 2007
Accepted: September 9, 2007
Published online: November 14, 2007
Colonization of stomach by H pylori is followed by a marked infiltration of the mucosa with polymorphonuclear leukocytes, macrophages, and lymphocytes that very often remains asymptomatic, but in some circumstances can lead to the development of gastroduodenal ulceration, gastric carcinoma, and mucosa-associated lymphoid tissue lymphoma. The molecular mechanisms by which H pylori triggers and maintains the local immune response are complex, but there is evidence that cytokines produced by both immune and non-immune cells contribute to amplify the ongoing inflammation. H pylori infection is associated with a marked mucosal induction of T helper (Th) type 1 and Th17-type cytokines that is governed by specific antigen-presenting cell-derived molecules, such as interleukin (IL)-12 and IL-23. In this paper, we will review the available data on the expression and role of IL-23 and IL-17 in H pylori-related gastritis.
-
Citation: Caruso R, Pallone F, Monteleone G. Emerging role of IL-23/IL-17 axis in
H pylori -associated pathology. World J Gastroenterol 2007; 13(42): 5547-5551 - URL: https://www.wjgnet.com/1007-9327/full/v13/i42/5547.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i42.5547
H pylori is a spiral-shaped Gram-negative flagellate bacterium that colonizes the human gastric mucosa and chronically infects more than half of the human population. Infection is inversely correlated with socioeconomic conditions. Most new H pylori infections occur in children, but the lack of specific H pylori-related clinical signs makes difficult to define the mode of transmission[1]. H pylori survives within the gastric mucus layer despite the acidic microenvironment, that limits the growth of most bacteria. This primarily relies upon the ability of H pylori to secrete a large amount of urease that breaks down urea into carbon dioxide and ammonia, the latter buffering its environment. Most H pylori organisms remain in the mucus layer, even though a small proportion adheres to the mucosal epithelial cells and rarely invades the mucosa[2]. Moreover, H pylori can inject into the epithelial cells bacterial products that modify epithelial cell functions[3].
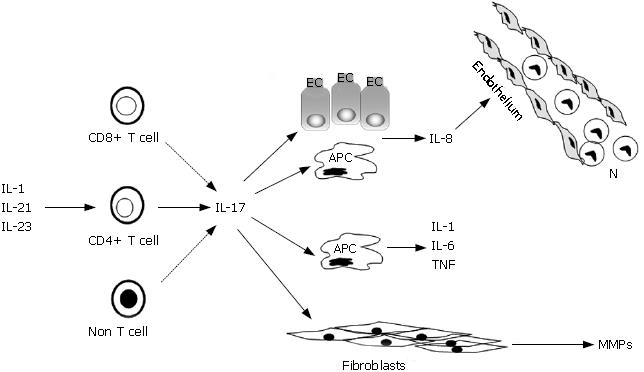
H pylori infection causes a marked infiltration of the gastric mucosa with neutrophils, macrophages, and lymphocytes. Most H pylori-infected patients are asymptomatic, but H pylori-driven gastritis can lead to the development of gastroduodenal ulcers, gastric carcinoma, and mucosa-associated lymphoid tissue lymphoma[4]. The level of inflammation increases the risk of disease, but it does not seem to influence which disease develops. In contrast, this is thought to be largely influenced by the pattern of gastric inflammation. In particular, antral gastritis is associated with increased stimulated acid production and predisposes to duodenal ulceration, while corpus-predominant or pan-gastritis is associated with reduced acid production and predisposes to gastric ulcer and gastric adenocarcinoma[5]. There is also evidence that the degree of gastric infiltration by neutrophils correlates with the development of gastro-duodenal ulcerations, and this is in part dependent on the release of damaging inflammatory mediators such as reactive oxygen species[6,7]. Because neutrophils are short-lived, they must be constantly recruited into the infected mucosa from circulation. Antigens released by H pylori can stimulate endothelial cells, macrophages and epithelial cells to make huge amounts of chemokines, such as interleukin (IL)-8 and growth-regulated oncogene-alpha, that produce a chemotactic gradient for the migration of neutrophils into the gastric mucosa[8-11]. It is also known that infections with specific H pylori strains that possess the cag pathogenicity island (cag+) induce significantly higher levels of chemokines than do cag-strains[12]. Both macrophages and epithelial cells also synthesize neutrophil-recruiting chemokines in response to lamina propria mononuclear cell (LPMC)-derived molecules. In this context, we and others have recently shown that IL-17, a key regulator of neutrophil chemotaxis, is produced in excess in H pylori-infected stomach[13-15]. By real-time PCR and Western blotting it was shown that IL-17 up-regulation occurs at both RNA and protein levels in H pylori-infected biopsies in comparison to uninfected biopsies either with or without gastritis[13,14]. Notably, among H pylori-positive patients, the gastric mucosa at the site of ulcers contains more IL-17 than the non-ulcerated mucosa of the antrum[15]. Several observations suggest that IL-17 plays a decisive role in the neutrophil recruitment to the H pylori-infected gastric mucosa. First, IL-17 levels correlate with the number of neutrophils infiltrating the Hp-infected mucosa[15]. Second, both gastric LPMC and epithelial cells express IL-17 receptors and are functionally capable of responding to IL-17 by secreting IL-8[14-16]. Consistently, conditioned media of gastric epithelial cells stimulated with IL-17 enhance the migration of peripheral blood neutrophils, and this effect is inhibitable by a blocking anti-IL-8, but not anti-IL-17 antibody (Figure 1)[14]. Functional analysis of intracellular pathways involved in the induction of IL-8 synthesis by IL-17 revealed that IL-17 activates ERK1/2 MAP kinases in gastric epithelial cells, and that pharmacologic blockade of this pathway significantly inhibits IL-8 secretion[16]. These findings are in line with the demonstration that activated ERK1/2 and IL-8 are more pronounced in gastric epithelial cells isolated from H pylori-infected biopsies in comparison to uninfected controls, and that neutralization of endogenous IL-17 in ex vivo cultures of H pylori-infected gastric biopsies down-regulates the expression of activated ERK1/2 and IL-8[16]. Finally, IL-17 expression positively correlates with IL-8 content in H pylori-colonized biopsies[15].
Besides its effects on IL-8 synthesis, IL-17 exerts additional immune-regulatory functions which could influence the magnitude and/or severity of H pylori-related gastritis. For example, IL-17 stimulates the production of IL-1, IL-6, and TNF-α by both immune and non-immune cells[17], and induces fibroblasts to make matrix metalloproteinases (MMPs)[18]. MMPs are a family of proteases that can cleave multiple components of the extracellular matrix, thereby contributing to the mucosal damage[19] (Figure 1).
IL-17 was originally named cytotoxic T lymphocyte-associated-8 (CTLA-8), subsequently IL-17, and more recently IL-17A, since it is one of six related members belonging to the IL-17 family (IL-17A-F)[20]. IL-17 was initially described at the message level as a product of human blood activated CD4+ memory T cells. Subsequent studies have shown that IL-17 can be also made by activated CD8+ T cells, TCRγδ+ T cells, and neutrophils[20]. More recently, it was shown that IL-17 is produced by a specific subset of CD4+ T cells, termed T helper (Th) 17-cells, that are distinct from, and antagonized by the classical Th1 or Th2 cells[21]. Th17-cells produce also, but to a lesser extent, TNF-α, IL-6, IL-17F, IL-22, and granulocyte macrophage-colony stimulating factor[22,23]. Flow-cytometry analysis of IL-17 production in gastric LPMC isolated from biopsies of H pylori-infected patients showed that CD4+ T cells are a major source of IL-17, even though CD8+ T cells and CD3-negative cells were also positive for IL-17 (Figure 1)[13].The molecular pathways governing the development of Th17-cells in humans have not been yet elucidated, but studies in murine systems indicate that Th-17 cell differentiation is driven by IL-6 and TGF-β1[24,25]. There is also evidence that expansion and survival of Th17-cells require additional factors, such as IL-23[25]. IL-23 is a heterodimeric protein that is composed by the p40 subunit of IL-12 and a specific subunit, termed IL-23/p19. The functional IL-23 heterodimer is produced by activated dendritic cells (DC), monocytes and macrophages[26]. We have recently shown that IL-23 protein is produced in excess in H pylori-colonized mucosa. RNA transcripts for both p40 and p19 subunits were also up-regulated in biopsies from H pylori-infected patients, indicating that IL-23 is regulated at the transcriptional level in this condition[13]. These results confirm and expand on data of previous studies showing that H pylori enhances IL-23 secretion by monocyte-derived DC[27], and that H pylori neutrophil-activating protein
(H pylori-NAP), a member of a broad super-family of ferritin-like proteins, induces IL-23 production by neutrophils and monocytes[28,29]. Functional studies also revealed that IL-23 enhances IL-17 synthesis by normal gastric LPMC, and that blockade of endogenous IL-23 activity in cultures of LPMC isolated from H pylori-infected biopsies down-regulates IL-17 production[13]. The exact molecular mechanism by which IL-23 regulates IL-17 in H pylori-infected mucosa remains to be ascertained. Notably, neutralization of endogenous IL-23 by a blocking anti-IL-23/p19 antibody in cultures of LPMC isolated from H pylori-infected biopsies attenuates the expression of active Stat3. Moreover, in normal gastric LPMC, exogenous IL-23 enhances the activation of Stat3, and pharmacologic inhibition of Stat3 suppresses IL-17 production induced by IL-23[13]. Taken together, these results suggest that Stat3 plays a key role in the IL-23-driven IL-17 production during H pylori infection. This well fits with the demonstration that Stat3 is essential for the induction and expansion of IL-17-producing cells in response to cytokine stimulation both in vitro and in vivo[30]. Such an effect could rely on the ability of Stat3 to bind the promoter of IL-17 gene and enhance its transcriptional activity[31], and/or favor the induction of RORγt, a master regulator of Th17-cell differentiation[32], and the expression of IL-23R.
IL-17 synthesis may be regulated by additional cytokines other than IL-23. IL-1R1-deficient mice fail to mount a robust Th17 response, and IL-1R1-deficient cells do not produce IL-17 in response to IL-23[33]. Since H pylori infection enhances the production of IL-1 at the gastric level[34], it is tempting to speculate that this cytokine may act in concert with IL-23 in enhancing IL-17. IL-17 synthesis is also increased by IL-15 in cultures of human and murine CD4+ T cells[35]. However, the fact that IL-15 expression is down-regulated in H pylori-infected biopsies argues against a role for IL-15 in the control of IL-17 production during H pylori-related gastritis[36]. Th17 cell differentiation is also enhanced by IL-21[37,38], a T-cell derived cytokine that is produced in excess in H pylori-colonized stomach[39].
During H pylori infection, there is a pronounced specific acquired immune response, characterized by generation of antibodies, and differentiation and activation of effector T cells. Although this later includes both a Th1 and a Th2 component, mucosal cytokine profiles imply Th1 predominance[40], and the number of cells producing interferon (IFN)-γ, the key Th1 cytokine, in the H pylori-infected human gastric mucosa correlates with the severity of gastritis[41]. Animal models also suggest that the extent of Th1 differentiation is important in pathogenesis. Mice with a predominant Th1 response develop more gastric inflammation during H pylori colonization than those with a Th2 response[42-43]. Gastric inflammation and atrophic changes are abrogated in the absence of IFN-γ[44], while IFN-γ infusion into mice, even in the absence of H pylori infection, induces pre-cancerous gastric atrophy, metaplasia and dysplasia[45]. IL-12-deficient mice have also reduced gastric inflammatory infiltration and are unable to clear H pylori infection[46].
Several virulence factors are reported to promote Th1 responses, including the plasticity region locus jhp0947-jhp0949 which is associated with duodenal ulcer disease[47] and the H pylori-NAP[29]. The Th1/Th2 balance is also influenced by phase-variable expression of Lewis blood-group antigens and genomic DNA recombination[48,49].
IL-12 is supposed to be one of the major Th1-inducing factors in H pylori-colonized gastric mucosa[50], even though IL-23 may contribute to expand the ongoing Th1 cell response[26]. Indeed, blockade of endogenous IL-23 by anti-IL23/p19 in cultures of LPMC isolated from H pylori-colonized biopsies reduces IFN-γ secretion, and stimulation of normal gastric LPMC with IL-23 enhances IFN-γ production. These data are in accordance with the demonstration that IL-23 activates Stat4 and enhances IFN-γ production in cultures of human and murine memory T cells[26], and that in two models of H. hepaticus-triggered T cell-dependent colitis, IL-23 enhances both IFN-γ and IL-17 responses that together synergize to trigger severe intestinal inflammation[51,52].
As pointed out above, H pylori is a major factor in the induction of gastritis and its progression to pre-neoplastic lesions and non-cardia gastric cancer. Despite the high prevalence of H pylori infection, the risk of gastric cancer in H pylori-infected patients is, however, estimated to be approximately 1%-3%. This indicates that infection per se is not sufficient to induce the progression to gastric neoplasia and that additional bacterial and host factors are required[2]. A detailed description of such factors is beyond the scope of this review. In this context it is, however, noteworthy that accumulating evidence would seem to suggest that IL-12 and IL-23 are important mediators in the process that links H pylori infection to gastric cancer. Indeed, polymorphisms of IL-12p40 and p35 genes enhance the risk of non-cardia gastric cancers in H pylori-infected patients[53]. Moreover, high levels of IL-23 have been documented in human gastric cancers[53]. Nonetheless, no functional study has so far mechanistically linked the activity of IL-12 and IL-23 to gastric cancer. Additionally, studies in murine models of epithelial cancers have shown that IL-23, but not IL-12, is essential for sustaining the tumor-promoting inflammatory process and counteracting the ability of cytotoxic CD8+ T cells to infiltrate tumors[54].
Although bacterial virulence factors are important in conditioning the outcome of the H pylori-driven infection, it is the host attempt to clear the bacterium that causes an exaggerated and inappropriately counter-regulated immune response that may eventually cause tissue damage. Emerging experimental evidence suggests that IL-23/IL-17 pathway is an important driving force the ongoing gastric inflammation in H pylori-infected patients. However, further studies will be required to establish the exact contribution of each of these cytokines in the H pylori-associated gastric pathology. The availability of strains of mice deficient either for IL-23 subunits or IL-17 should provide valuable models to specifically address these issues.
S- Editor Liu Y L- Editor Rippe RA E- Editor Li JL
| 1. | Go MF. Review article: natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16 Suppl 1:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 276] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 2. | Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 612] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 3. | Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791-5795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 933] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 4. | Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol. 2000;54:615-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 398] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 5. | Robinson K, Argent RH, Atherton JC. The inflammatory and immune response to Helicobacter pylori infection. Best Pract Res Clin Gastroenterol. 2007;21:237-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Davies GR, Simmonds NJ, Stevens TR, Sheaff MT, Banatvala N, Laurenson IF, Blake DR, Rampton DS. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut. 1994;35:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 303] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Zhang QB, Nakashabendi IM, Mokhashi MS, Dawodu JB, Gemmell CG, Russell RI. Association of cytotoxin production and neutrophil activation by strains of Helicobacter pylori isolated from patients with peptic ulceration and chronic gastritis. Gut. 1996;38:841-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Innocenti M, Thoreson AC, Ferrero RL, Strömberg E, Bölin I, Eriksson L, Svennerholm AM, Quiding-Järbrink M. Helicobacter pylori-induced activation of human endothelial cells. Infect Immun. 2002;70:4581-4590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Sieveking D, Mitchell HM, Day AS. Gastric epithelial cell CXC chemokine secretion following Helicobacter pylori infection in vitro. J Gastroenterol Hepatol. 2004;19:982-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Sharma SA, Tummuru MK, Miller GG, Blaser MJ. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995;63:1681-1687. [PubMed] |
| 11. | de Jonge R, Kusters JG, Timmer MS, Gimmel V, Appelmelk BJ, Bereswill S, van Vliet AH, Meuwissen SG, Kist M, Vandenbroucke-Grauls CM. The role of Helicobacter pylori virulence factors in interleukin production by monocytic cells. FEMS Microbiol Lett. 2001;196:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Nilsson C, Sillén A, Eriksson L, Strand ML, Enroth H, Normark S, Falk P, Engstrand L. Correlation between cag pathogenicity island composition and Helicobacter pylori-associated gastroduodenal disease. Infect Immun. 2003;71:6573-6581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Caruso R, Fina D, Paoluzi AO, Del Vecchio Blanco G, Stolfi C, Fantini MC, Rizzo A, Andrei F, Peluso I, Caprioli F. IL-23 enhances IL-17 production in H pylori-infected gastric mucosa. Gastroenterology. 2007;4 Suppl 2:A-219. |
| 14. | Luzza F, Parrello T, Monteleone G, Sebkova L, Romano M, Zarrilli R, Imeneo M, Pallone F. Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. J Immunol. 2000;165:5332-5337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 214] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Mizuno T, Ando T, Nobata K, Tsuzuki T, Maeda O, Watanabe O, Minami M, Ina K, Kusugami K, Peek RM. Interleukin-17 levels in Helicobacter pylori-infected gastric mucosa and pathologic sequelae of colonization. World J Gastroenterol. 2005;11:6305-6311. [PubMed] |
| 16. | Sebkova L, Pellicanò A, Monteleone G, Grazioli B, Guarnieri G, Imeneo M, Pallone F, Luzza F. Extracellular signal-regulated protein kinase mediates interleukin 17 (IL-17)-induced IL-8 secretion in Helicobacter pylori-infected human gastric epithelial cells. Infect Immun. 2004;72:5019-5026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513-3521. [PubMed] |
| 18. | Bamba S, Andoh A, Yasui H, Araki Y, Bamba T, Fujiyama Y. Matrix metalloproteinase-3 secretion from human colonic subepithelial myofibroblasts: role of interleukin-17. J Gastroenterol. 2003;38:548-554. [PubMed] |
| 19. | Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274:21491-21494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3230] [Cited by in RCA: 3153] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 20. | Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1754] [Cited by in RCA: 1817] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 21. | Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 978] [Cited by in RCA: 982] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 22. | Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3074] [Cited by in RCA: 3359] [Article Influence: 168.0] [Reference Citation Analysis (0)] |
| 23. | Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1417] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 24. | Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5474] [Article Influence: 288.1] [Reference Citation Analysis (0)] |
| 25. | Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2372] [Cited by in RCA: 2489] [Article Influence: 131.0] [Reference Citation Analysis (0)] |
| 26. | Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2046] [Cited by in RCA: 2109] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 27. | Mitchell P, Germain C, Fiori PL, Khamri W, Foster GR, Ghosh S, Lechler RI, Bamford KB, Lombardi G. Chronic exposure to Helicobacter pylori impairs dendritic cell function and inhibits Th1 development. Infect Immun. 2007;75:810-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | D'Elios MM, Amedei A, Cappon A, Del Prete G, de Bernard M. The neutrophil-activating protein of Helicobacter pylori (HP-NAP) as an immune modulating agent. FEMS Immunol Med Microbiol. 2007;50:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Amedei A, Cappon A, Codolo G, Cabrelle A, Polenghi A, Benagiano M, Tasca E, Azzurri A, D'Elios MM, Del Prete G. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J Clin Invest. 2006;116:1092-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 252] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 30. | Cho ML, Kang JW, Moon YM, Nam HJ, Jhun JY, Heo SB, Jin HT, Min SY, Ju JH, Park KS. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006;176:5652-5661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 254] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 31. | Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O'Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci USA. 2006;103:8137-8142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 531] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 32. | Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3769] [Cited by in RCA: 4134] [Article Influence: 217.6] [Reference Citation Analysis (0)] |
| 33. | Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 760] [Cited by in RCA: 810] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 34. | Noach LA, Bosma NB, Jansen J, Hoek FJ, van Deventer SJ, Tytgat GN. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994;29:425-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 350] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 35. | Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170:2106-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 414] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 36. | Luzza F, Parrello T, Monteleone G, Sebkova L, Imeneo M, La Vecchia A, Maletta M, Docimo C, Pallone F. Changes in the mucosal expression of interleukin 15 in Helicobacter pylori-associated gastritis. FEMS Immunol Med Microbiol. 1999;24:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1499] [Cited by in RCA: 1469] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 38. | Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1167] [Cited by in RCA: 1179] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 39. | Caruso R, Fina D, Peluso I, Fantini MC, Tosti C, Del Vecchio Blanco G, Paoluzi OA, Caprioli F, Andrei F, Stolfi C. IL-21 is highly produced in Helicobacter pylori-infected gastric mucosa and promotes gelatinases synthesis. J Immunol. 2007;178:5957-5965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | D'Elios MM, Manghetti M, De Carli M, Costa F, Baldari CT, Burroni D, Telford JL, Romagnani S, Del Prete G. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962-967. [PubMed] |
| 41. | Lehmann FS, Terracciano L, Carena I, Baeriswyl C, Drewe J, Tornillo L, De Libero G, Beglinger C. In situ correlation of cytokine secretion and apoptosis in Helicobacter pylori-associated gastritis. Am J Physiol Gastrointest Liver Physiol. 2002;283:G481-G488. [PubMed] |
| 42. | Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn SJ. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 212] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, Nagler-Anderson C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 359] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 44. | Smythies LE, Waites KB, Lindsey JR, Harris PR, Ghiara P, Smith PD. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J Immunol. 2000;165:1022-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 269] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 45. | Cui G, Houghton J, Finkel N, Carlson J, Wang TC. IFN-gamma infusion induces gastric atrophy, metaplasia and dysplasia in the absence of Helicobacter infection-a role for immune response in Helicobacter disease. Gastroenterology. 2003;124 Suppl 1:A19. |
| 46. | Akhiani AA, Pappo J, Kabok Z, Schön K, Gao W, Franzén LE, Lycke N. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. J Immunol. 2002;169:6977-6984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 179] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 47. | de Jonge R, Kuipers EJ, Langeveld SC, Loffeld RJ, Stoof J, van Vliet AH, Kusters JG. The Helicobacter pylori plasticity region locus jhp0947-jhp0949 is associated with duodenal ulcer disease and interleukin-12 production in monocyte cells. FEMS Immunol Med Microbiol. 2004;41:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Bergman MP, Engering A, Smits HH, van Vliet SJ, van Bodegraven AA, Wirth HP, Kapsenberg ML, Vandenbroucke-Grauls CM, van Kooyk Y, Appelmelk BJ. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J Exp Med. 2004;200:979-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 243] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 49. | Robinson K, Loughlin MF, Potter R, Jenks PJ. Host adaptation and immune modulation are mediated by homologous recombination in Helicobacter pylori. J Infect Dis. 2005;191:579-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Pellicanò A, Sebkova L, Monteleone G, Guarnieri G, Imeneo M, Pallone F, Luzza F. Interleukin-12 drives the Th1 signaling pathway in Helicobacter pylori-infected human gastric mucosa. Infect Immun. 2007;75:1738-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473-2483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 667] [Cited by in RCA: 647] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 52. | Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485-2494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 475] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 53. | Navaglia F, Basso D, Zambon CF, Ponzano E, Caenazzo L, Gallo N, Falda A, Belluco C, Fogar P, Greco E. Interleukin 12 gene polymorphisms enhance gastric cancer risk in H pylori infected individuals. J Med Genet. 2005;42:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 772] [Article Influence: 40.6] [Reference Citation Analysis (0)] |