Published online Nov 7, 2007. doi: 10.3748/wjg.v13.i41.5540
Revised: August 18, 2007
Accepted: August 31, 2007
Published online: November 7, 2007
Infection with tapeworms is a major problem in many parts of the world. Patients may be asymptomatic or have a significant morbidity depending on the species. Infection with Taenia species is sometimes found by expulsion of eggs or proglottids in stool. Species specific diagnosis of Taenia is difficult, but possible. We present a case of Taenia saginata incidentally discovered, and risk factors for transmission, diagnosis, symptoms, and treatment.
-
Citation: Patel NM, Tatar EL. Unusual colonoscopy finding:
Taenia saginata proglottid. World J Gastroenterol 2007; 13(41): 5540-5541 - URL: https://www.wjgnet.com/1007-9327/full/v13/i41/5540.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i41.5540
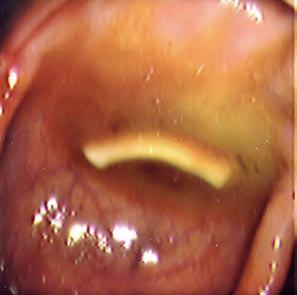
A 63-year-old Lebanese male presented for routine surveillance colonoscopy of polyps. He denied abdominal pain, hematochezia, weight loss, or change in bowel habits. Physical examination and laboratory studies including a complete blood count were normal. While withdrawing the scope, the following item was seen (Figure 1).
The linear white object represented a parasite. On endoscopic examination, it was found to move within the colon. When retrieved and further analyzed, it was found to be a proglottid of Taenia saginata, the beef tapeworm. The scolex and majority of the worm are present in the small bowel, with the head usually residing in the jejunum or ileum. Each segment, known as a proglottid, has a complete set of reproductive organs. The adult worm may have hundreds to thousands of proglottids. The more distal the proglottids are, the more mature they are, containing an increasing number of eggs. Taenia species bud off distal segments from the rest of the body that are passed through the feces. The mature T. saginata tapeworm can reach 4-6 meters or more in length, and has 1000-2000 proglottids. The scolex has 4 suckers, but no hooks. In contrast, the mature T. solium, or pork tapeworm can reach 2-4 meters or more in length, and has 800-1000 proglottids. The scolex has 4 suckers and a small rostellum with a double crown of 25-30 small hooks[1].
Finding eggs or proglottids in the stool makes the diagnosis of Taenia infection. The eggs of T. saginata are indistinguishable from T. solium, and a species specific diagnosis requires examination of a proglottid segment. The microscopic differentiation of gravid T. saginata proglottids (usually more than 15 lateral uterine branches, vaginal sphincter muscle, and two ovarian lobes) and T. solium proglottids (usually 5-10 uterine branches on each side, vaginal sphincter muscle absent, one ovarian lobe) is possible. This is the only practical method that can be used in a basic laboratory if only gravid proglottids passed out in stool are present for diagnosis. The presence of a vaginal sphincter muscle in the proglottid can identify the organism as T. saginata. The presence of 2 ovarian lobes is also a specific trait of T. saginata. Following antihelminth therapy, the scolex is shed and in some cases may be retrieved. The absence of hooks on the scolex is a characteristic of T. saginata[2]. If findings are doubtful, the differentiation should be done in a specialized helminthological laboratory by enzyme electrophoresis, polymerase chain reaction (PCR), or various immunological assays[3].
The beef tapeworm is a common infection of both humans and cattle throughout the world, particularly in areas wherever beef is eaten. Areas of high prevalence are sub-Saharan African, southeast Asia, and the Middle East. Infection is associated with eating raw beef, poor sanitation, and allowing cattle on pastures fertilized by sewage sludge contaminated with human feces[4]. Lifecycles for Taenia involve two mammalian hosts, a carnivorous or omnivorous host, and a herbivorous intermediate host. In the tapeworm life cycle, humans are the final host and infections are acquired by ingesting raw or undercooked meat containing the cysticercus stage in a host capsule. When the cysticercus stage reaches the stomach, proteolytic enzymes start dissolving the capsule. In the small intestine, the cysticercus is stimulated to evaginate. The scolex attaches to the intestinal mucosa by means of 4 suckers and starts growing into a mature tapeworm. Mature tapeworms have been known to live in the human gastrointestinal tract for up to 25 years[1]. Upon further questioning, our patient noted that he frequently ate raw beef in his native country.
Most patients who carry an adult T. saginata tapeworm are asymptomatic. The only symptom found in such a patient may be the spontaneous passage of proglottids. Nonspecific symptoms such as abdominal discomfort, epigastric pain, nausea, vomiting, diarrhea, and weight loss are known to occur[1], but these symptoms are rare. Obstruction of the appendix, pancreatic duct, or common bile duct by proglottid segments has been reported[1]. The occurrence of weight loss and malnutrition is extremely rare. B12 deficiency and megaloblastic anemia are not seen in Taeniasis, and associated with Diphyllobothrium species found in fish species. B12 deficiency is thought to occur as a result of the ability to compete with the human host for vitamin B12.
While the adult form of T. saginata generally does not lead to serious complications, juvenile forms of other tapeworm species may lead to such complications. Ingested ova from T. solium, the pork tapeworm, can form cysticercosis in the brain, subcutaneous tissue, skeletal muscle, eye, or other organs causing a significant morbidity. The life cycle of T. solium includes pigs that are the intermediate host because they develop the larval stage and transmit the parasite when human beings ingest insufficiently cooked pork[5]. It is endemic in Mexico, Latin America, tropical Africa, southeast Asia, the Philippines, and the Indian subcontinent[2]. Ova from Echinococcus, a tapeworm associated with canines, can also cause cysticercosis with a predominance for occurrence in the liver. There is no evidence that cysticerci can develop in humans as a result of T. saginata infection[6].
The treatment of choice in intestinal Taeniasis (T. saginata and T. solium) is praziquantel, a synthetic heterocyclic isoquinolone-pyrazine derivative. A single dose of 5 to 10 mg/kg has an efficacy of greater than 95%. Praziquantel induces ultrastructural changes in the teguments of parasites, resulting in increased permeability to calcium ions[7]. Calcium ions accumulate in parasite cytosol causing muscular contractions and ultimate paralysis of the worm. Additionally, this exposes parasite antigens to the host immune response. The ultimate response is dislodgement of worms from their intestinal sites and subsequent expulsion by peristalsis. For successful treatment, the scolex must be destroyed, and eliminated because residual scolex can result in regrowth. Albendazole or praziquantel can be used in the treatment of cysticercosis.
For people in high risk communities, primary prevention of Taeniasis is the removal of intermediate hosts such as cattle and pigs from the parasite's life cycle by eliminating exposure to raw sewage[1] and adequately cooking meats before consumption.
S- Editor Liu Y L- Editor Wang XL E- Editor Liu Y
| 1. | Collier L, Cox F, Topley WW. Topley and Wilson's Microbiology and Microbial Infections: Parasitology, Vol 5. London: Edward Arnold Publishers 1998; 521-537. |
| 2. | Murray PR. Manual of Clinical Microbiology. 7th ed. Washington DC: ASM Press 1999; 1432-1437. |
| 3. | Raether W, Hänel H. Epidemiology, clinical manifestations and diagnosis of zoonotic cestode infections: an update. Parasitol Res. 2003;91:412-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Joklik WK, Willett HP, Amos DB, Wilfert CM. Zinnser Microbiology. 20th ed. Norwalk: Appleton & Lange 1992; 1208-1210. |
| 5. | Flisser A. Where are the tapeworms? Parasitol Int. 2006;55 Suppl:S117-S120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Hoberg EP. Taenia tapeworms: their biology, evolution and socioeconomic significance. Microbes Infect. 2002;4:859-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Hardman JG, Limbird LE. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York: McGraw Hill 2006; 1073-1093. |