Published online Nov 7, 2007. doi: 10.3748/wjg.v13.i41.5471
Revised: July 28, 2007
Accepted: August 27, 2007
Published online: November 7, 2007
AIM: To investigate the systemic hemodynamic effects of two surgical procedures largely employed for treatment of schistosomal portal hypertension.
METHODS: Thirty-six patients undergoing elective surgical treatment of portal hypertension due to hepatosplenic mansonic schistosomiasis were prospectively evaluated. All patients were subjected to preoperative pulmonary artery catheterization; 17 were submitted to esophagogastric devascularization and splenectomy (EGDS) and 19 to distal splenorenal shunt (DSRS). The systemic hemodynamic assessment was repeated 4 d after the surgical procedure.
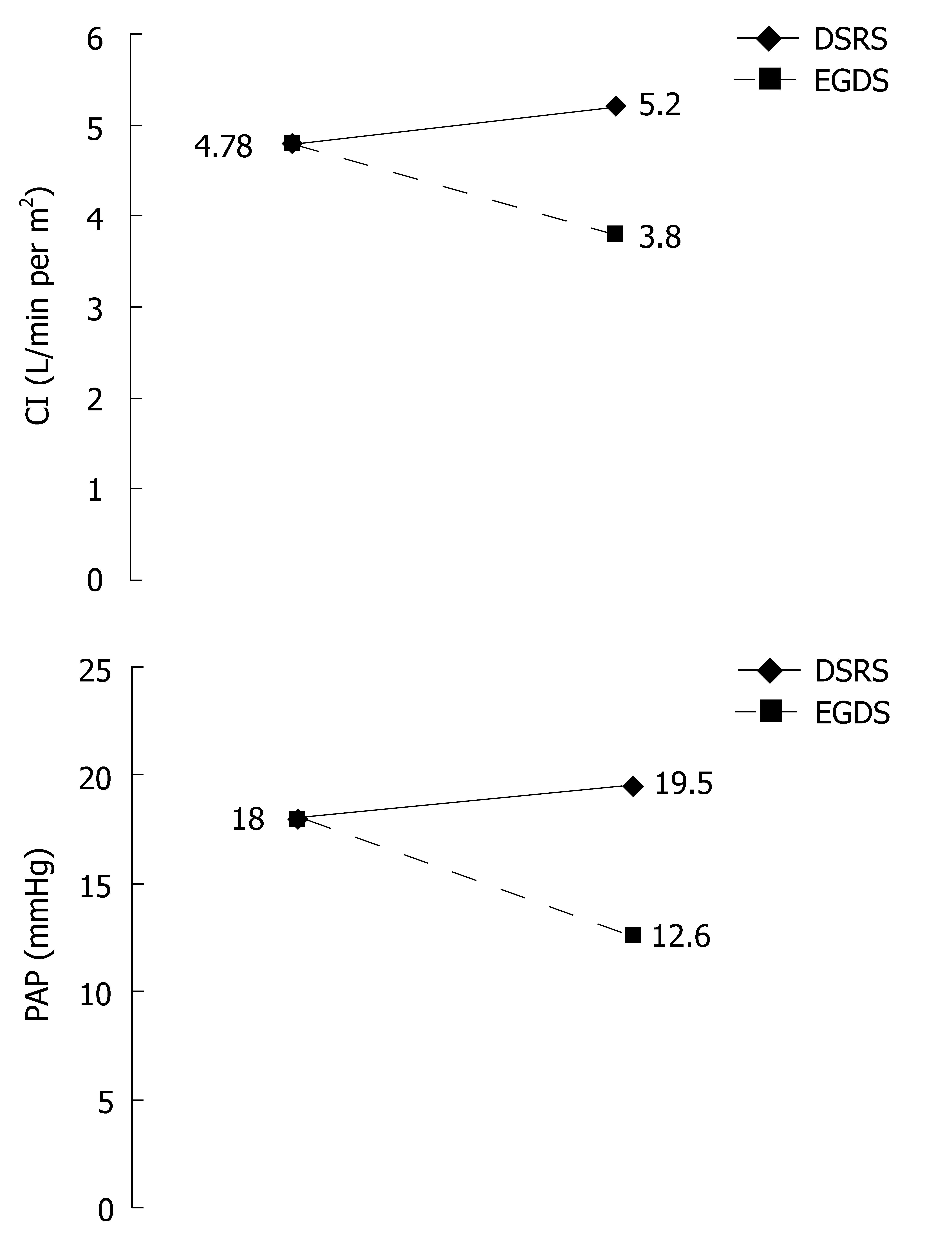
RESULTS: Preoperative evaluation revealed (mean ± SD) an increased cardiac index (4.78 ± 1.13 L/min per m2), associated with a reduction in systemic vascular resistance index (1457 ± 380.7 dynes.s/cm5.m2). The mean pulmonary artery pressure (18 ± 5.1 mmHg) as well as the right atrial pressure (7.9 ± 2.5 mmHg) were increased, while the pulmonary vascular resistance index (133 ± 62 dynes.s/cm5.m2) was decreased. Four days after EGDS, a significant reduction in cardiac index (3.80 ± 0.4 L/min per m2, P < 0.001) and increase in systemic vascular resistance index (1901.4 ± 330.2 dynes.s/cm5.m2, P < 0.001) toward normal levels were observed. There was also a significant reduction in pulmonary artery pressure (12.65 ± 4.7 mmHg, P < 0.001) and no significant changes in the pulmonary vascular resistance index (141.6 ± 102.9 dynes.s/cm5.m2). Four days after DSRS, a non-significant increase in cardiac index (5.2 ± 0.76 L/min per m2) and systemic vascular resistance index (1389 ± 311 dynes.s/cm5.m2) was observed. There was also a non-significant increase in pulmonary artery pressure (19.84 ± 5.2 mmHg), right cardiac work index (1.38 ± 0.4 kg.m/m2) and right ventricular systolic work index (16.3 ± 6.3 g.m/m2), without significant changes in the pulmonary vascular resistance index (139.7 ± 67.8 dynes.s/cm5.m2).
CONCLUSION: The hyperdynamic circulatory state observed in mansonic schistosomiasis was corrected by EGDS, but was maintained in patients who underwent DSRS. Similarly, the elevated mean pulmonary artery pressure was corrected after EGDS and maintained after DSRS. EGDS seems to be the most physiologic surgery for patients with schistosomal portal hypertension.
- Citation: de Cleva R, Herman P, D'albuquerque LAC, Pugliese V, Santarem OL, Saad WA. Pre- and postoperative systemic hemodynamic evaluation in patients subjected to esophagogastric devascularization plus splenectomy and distal splenorenal shunt: A comparative study in schistomomal portal hypertension. World J Gastroenterol 2007; 13(41): 5471-5475
- URL: https://www.wjgnet.com/1007-9327/full/v13/i41/5471.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i41.5471
In Brazil, mansonic schistosomiasis is an endemic disease, and its hepatosplenic form, which is characterized by presinusoidal portal hypertension with preserved liver function and marked splenomegaly, is a major cause of portal hypertension[1-3]. Upper digestive tract hemorrhage due to esophageal varices rupture is the most feared complication[4].
The development of portal hypertension, regardless of its etiology, is a consequence of increased vascular resistance, mostly due to an architectural distortion of the liver parenchyma secondary to fibrosis, but also due to a diminished endothelial nitric oxide release from the hepatic endothelium[5]. Increased portal venous inflow due to mesenteric arteriolar vasodilatation, and determined by increased levels of vasodilators, also contributes to portal pressure increase[6].
The pathophysiology of portal hypertension in hepatosplenic mansonic schistosomiasis also displays a systemic hyperdynamic state[2]. We have previously reported that this hyperdynamic circulatory state seems to be corrected in the intraoperative period during esophagogastric devascularization and splenectomy (EGDS)[7]. However, to the best of our knowledge, there are no data in the literature regarding postoperative systemic hemodynamics after surgical treatment of schistosomal portal hypertension.
The purpose of this study was to prospectively investigate the postoperative systemic hemodynamic effects in two different surgical procedures largely employed for treatment of schistosomal portal hypertension.
Thirty-six patients with portal hypertension and a history of previous upper digestive tract bleeding, due to esophageal varices rupture secondary to hepatosplenic mansonic schistosomiasis, were prospectively studied before and after elective surgical treatment between June 1998 and March 2005. Eighteen patients were male and eighteen female, with a mean age of 39 (range 22-56) years. Laboratory data and arterial blood gases are expressed in Table 1. Transthoracic echocardiography was performed in all patients before surgery. The Hospital Ethics Committee approved the study protocol, and all patients signed their informed consent. Immediately before surgery, patients underwent a right internal jugular vein puncture with the introduction of a pulmonary artery catheter (Edwards Swan-Ganz TM, caliber 7F, model 93A-131H; Baxter Corporation, USA) for invasive systemic hemodynamic assessment. Patients were randomized for two different elective surgical procedures: 17 were subjected to esophagogastric devascularization and splenectomy (EGDS) and 19 to distal splenorenal shunt (DSRS). A mean pulmonary artery pressure greater than 25 mmHg was considered as an absolute contraindication for DSRS.
| Mean ± SD | Normal values | |
| ALT (IU/L) | 31.7 ± 16.8 | 7-45 |
| AST (IU/L) | 31.6 ± 20.5 | 7-45 |
| Gamma GT (IU/L) | 46.2 ± 25.5 | 7-50 |
| ALP (IU/L) | 118.5 ± 46.3 | 60-122 |
| BUN (mg/dL) | 25.5 ± 5.75 | 10-50 |
| Cr (mg/dL) | 0.78 ± 0.16 | 0.6-1.4 |
| TP (g/dL) | 7.45 ± 0.71 | 6-8 |
| ALB (g/dL) | 4.18 ± 0.48 | 3.5-5.0 |
| PT (s) | 14.2 ± 2.9 | 14 ± 2 |
| PTT (s) | 29.8 ± 6.26 | 30 ± 2 |
| TBIL (mg%) | 1.16 ± 0.73 | 1.4 |
| IBIL (mg%) | 0.79 ± 0.67 | 0.8 |
| Hb (g/dL) | 11.2 ± 2.4 | 12-18 |
| Ht (%) | 34.4 ± 7.2 | 36-54 |
| WBC (103/mm3) | 3.63 ± 2.2 | 4-10 |
| PLT (103/mm3) | 88.74 ± 56.24 | 150-400 |
| pH | 7.41 ± 0.05 | 7.37-7.44 |
| pO2 (mmHg) | 91.4 ± 6.5 | 80-100 |
| pCO2 (mmHg) | 34.9 ± 3.2 | 34-45 |
| SaO2 (%) | 97.1 ± 0.95 | 96-98 |
EGDS consisted of ligation of the splenic artery close to the body of the pancreas, followed by splenectomy and devascularization of the distal 5-7 cm of the esophagus, and of the upper two thirds of the stomach proximal to the incisura angularis. DSRS consisted of dissection of the splenic vein from the splenic hilum until its junction with superior mesenteric vein, and ligating all small vessels between the pancreas and the splenic vein (splenopancreatic disconnection). Left renal vein anterior and superior surfaces were dissected, the splenic vein was transected near it's junction with the superior mesenteric vein, and an anastomosis between the splenic and renal veins was performed with a running suture. No immediate complications were observed and there was no intra- or postoperative mortality.
Four days after surgery, when the surgical effects had worn off, the systemic hemodynamic assessment was repeated and the pulmonary artery catheter was removed. There were no complications related to pulmonary artery catheterization.
Statistical analysis was accomplished by the paired t test, and P < 0.01 was considered as statistically significant, with a 99% confidence interval.
The results of the transthoracic Doppler echocardiography are shown in Table 2. No ventricular hypertrophy or segmental contraction abnormality was observed, and no patients presented valvular lesions or pericardial effusions. All patients presented normal systolic and diastolic ventricular function. In two patients, echocardiography revealed an estimated pulmonary artery pressure of 60 and 40 mmHg, respectively, accompanied by a discrete dilatation of the right ventricle. These two patients were subjected to EGDS.
| Preop (mean ± SD) | Normal range | |
| Ao | 30.7 ± 1.9 | 20-35 mm |
| LA | 38.1 ± 4.6 | 20-40 mm |
| LVDD | 49.7 ± 3.9 | 35-55 mm |
| FDV | 125.4 ± 29.6 | 50-150 mL |
| LVSD | 30.9 ± 2.8 | 20-35 mm |
| SV | 30.1 ± 8.1 | 50-150 mL |
| DD | 37.6 ± 3.3 | 30%-40% |
| EF | 75.9 ± 4.5 | 65%-80% |
| Se | 8.9 ± 0.6 | 7-11 mm |
| Pw | 8.4 ± 0.5 | 7-11 mm |
| V/M | 65.1 ± 6.8 | 45%-75% |
Pre- and postoperative hemodynamic evaluation data are shown in Table 3 and Figure 1. Preoperative hemodynamic evaluation revealed an increased mean cardiac index (4.78 ± 1.13 L/min per m2) and a reduction in the systemic vascular resistance index (1457 ± 380.7 dynes.s/cm5.m2). The systolic index (60.24 ± 12.8 mL/beats per m2), left cardiac work index (6.14 ± 1.43 kg.m/m2), left ventricle systolic work index (76.6 ± 17.8 g.m/m2), right cardiac work index (1.22 ± 0.5 kg.m/m2) and right ventricle systolic work index (15.25 ± 6.4 g.m/m2) were all increased. Heart rate (80.2 ± 11.4 beats/min), mean arterial blood pressure (91.4 ± 12.5 mmHg) and pulmonary capillary wedge pressure (10.2 ± 2.7 mmHg) were all within normal limits. Mean pulmonary artery pressure (18 ± 5.1 mmHg), as well as right atrial pressure (7.9 ± 2.5 mmHg), was increased, while the pulmonary vascular resistance index (133 ± 62 dynes.s/cm5.m2) was decreased.
| Preop | EGDS | DSRS | Normal values | |
| HR | 80.2 ± 11.4 | 83.1 ± 10.6 | 86.7 ± 17.9 | 80-100 beats/min |
| MABP | 91.4 ± 12.5 | 93.6 ± 14.4 | 92.36 ± 13.75 | 80-100 mmHg |
| RAP | 7.9 ± 2.5 | 7 ± 2.4 | 7.26 ± 2.4 | 0-7 mmHg |
| PCWP | 10.2 ± 2.7 | 9.1 ± 3 | 9.55 ± 2 | 8-12 mmHg |
| PAP | 18 ± 5.1 | 12.65 ± 4.7d | 19.84 ± 5.2 | 12-15 mmHg |
| CI | 4.78 ± 1.13 | 3.8 ± 0.4d | 5.2 ± 0.76 | 2.5-4 L/min per m2 |
| SI | 60.24 ± 12.8 | 46.2 ± 8.6d | 59.64 ± 10.5 | 41-51 mL/beat per m2 |
| SVRI | 1457 ± 380.7 | 1901.4 ± 330.2d | 1389 ± 311 | 1970-2390 dynes.s/cm5.m2 |
| PVRI | 133 ± 62 | 141.65 ± 102.9 | 139.7 ± 67.8 | 225-315 dynes.s/cm5.m2 |
| LCWI | 6.14 ± 1.43 | 4.9 ± 0.7b | 6.94 ± 1.3 | 3.4-4.2 kg.m/m2 |
| LVSWI | 76.6 ± 17.8 | 59 ± 12.6b | 82.65 ± 17 | 50-62 g.m/m2 |
| RCWI | 1.22 ± 0.5 | 0.79 ± 0.4b | 1.31 ± 0.35 | 0.54-0.60 kg.m/m2 |
| RVSWI | 15.25 ± 6.4 | 9.45 ± 4.8b | 16.3 ± 6.3 | 7.9-9.7 g.m/m2 |
Four days after EGDS, there was a significant decrease in cardiac index (3.8 ± 0.4 L/min per m2) and a significant increase in systemic vascular resistance index (1901.4 ± 330.2 dynes.s/cm5.m2) toward normal levels. The systolic index (46.2 ± 8.6 mL/beats per m2), left cardiac work index (4.9 ± 0.7 kg.m/m2) and left ventricle systolic work index (59 ± 12.6 g.m/m2) also significantly decreased toward normal levels. There was no significant alteration in heart rate (83.1 ± 10.6 beats/min), mean arterial blood pressure (93.6 ± 14.4 mmHg), pulmonary capillary wedge pressure (9.1 ± 3 mmHg) and right atrial pressure (7 ± 2.4 mmHg). There was also a significant reduction in pulmonary artery pressure (12.65 ± 4.7 mmHg), right cardiac work index (0.79 ± 0.4 kg.m/m2), right ventricle systolic work index (9.45 ± 4.8 g.m/m2), and a non-significant increase in pulmonary vascular resistance index (141.65 ± 102.9 dynes.s/cm5.m2).
Four days after DSRS, there was a non-significant increase in cardiac index (5.2 ± 0.76 L/min per m2), and a non-significant decrease in systemic vascular resistance index (1389 ± 311 dynes.s/cm5.m2). The systolic index (59.64 ± 10.5 mL/beats per m2), left cardiac work index (6.94 ± 1.3 kg.m/m2) and left ventricle systolic work index (82.65 ± 17 g.m/m2) remained above normal levels. There were non-significant increases in heart rate (86.7 ± 17.9 beats/min) and mean arterial blood pressure (92.36 ± 13.75 mmHg), and non-significant decreases in pulmonary capillary wedge pressure (9.55 ± 2 mmHg) and right atrial pressure (7.26 ± 2.4 mmHg). In addition, there were non-significant increases in pulmonary artery pressure (19.84 ± 5.2 mmHg), right cardiac work index (1.31 ± 0.35 kg.m/m2), and right ventricle systolic work index (16.3 ± 6.3 g.m/m2), with no significant alteration in pulmonary vascular resistance index (139.7 ± 67.8 dynes.s/cm5.m2).
Hemodynamic changes after the surgical treatment of schistosomal portal hypertension with disconnection or shunt procedures may contribute to the understanding of the hyperdynamic circulation observed in these patients with portal hypertension and preserved liver function. Twenty-nine patients (80.5%) showed an elevated cardiac index in the preoperative evaluation, which characterized a hyperdynamic circulatory state[8,9]. Although easily clinically recognized in patients with cirrhosis by peripheral arterial vasodilatation, hypotension and tachycardia, which are usually related to progressive liver failure, in schistosomal patients, these alterations are completely absent because they are less intense and liver function is preserved[10].
We have previously demonstrated that hepatosplenic schistosomiasis presents mild pulmonary hypertension and induces a hyperdynamic circulatory state, which is corrected after EGDS[2,7]. We have suggested that these changes are correlated with the portosystemic collateral circulation, especially as a consequence of splanchnic hyperflow[7]. Our group, as do Wattanasirichaigoon et al[8], believes that portosystemic collateral circulation mimics an arteriovenous fistula, in which the high-pressure portal blood connects with the lower pressure systemic venous circulation, which decompresses the portal circulation, but increases portal blood flow. As portal blood flow increases, so does collateral flow, and it is almost totally shunted with the systemic circulation. These observations should be considered particularly in patients with hepatosplenic schistosomiasis, in whom large splenomegaly induces splenic hyperflow, which plays an important role in the origin and maintenance of hyperdynamic circulation[3]. In the present study, 15 patients subjected to EGDS (88.2%) presented normalization of cardiac and systemic vascular resistance indexes, which suggested that EGDS corrected the hyperdynamic circulation. In cirrhosis, physical exercise and pharmacological stress determine an increase in left ventricular end diastolic pressure and a fall in cardiac stroke index and left ventricular ejection fraction, which indicates an abnormal ventricular response[9,11,12]. In these patients, the reduced vascular resistance may mask left ventricular failure. In contrast, the correction of hyperdynamic circulation without elevation of filling pressure, and the normal pre-operative echocardiographic parameters strongly suggest an absence of cardiomyopathy in patients with portal hypertension due to hepatosplenic mansonic schistosomiasis.
On the other hand, patients subjected to DSRS maintained a hyperdynamic circulation. These observations suggested that splenic flow through the venous shunt maintained a low-resistance circuit and hence, the hyperdynamic circulation. These findings are corroborated by the increases in heart rate, systolic, left cardiac work and left ventricle systolic work indexes observed in these patients. Studies that assess the hemodynamic pattern in a later period after EGDS or DSRS are necessary to confirm the findings of our study.
Portopulmonary hypertension (PPHTN) is defined as an increase in mean pulmonary artery pressure greater than 25 mmHg, increased pulmonary vascular resistance (greater than 120 dynas/cm5), and pulmonary capillary wedge pressure lower than 15 mmHg, in the presence of portal hypertension[13-15]. There are several mechanisms proposed for its development, including an increased production of vasoconstrictors[16-18], increased pulmonary blood flow leading to vascular endothelial damage and remodeling[19], excess of pulmonary vascular volume[20], cirrhotic cardiomyopathy with myocardial thickening and diastolic dysfunction[21], and in situ microthrombosis[22]. Interestingly, to date there are no data showing any correlation between the extent of PPHTN and the intensity of portal pressure, severity of liver disease or degree of shunting[23].
Arterial blood gases and left (systolic and diastolic) ventricular function were within normal limits and, pulmonary capillary wedge pressure was < 15 mmHg in all patients during the hemodynamic study, which provided evidence of normal cardiopulmonary function.
In the present study, 23 patients (63.8%) presented with pulmonary artery pressure greater than 15 mmHg; 13 (36.1%) between 15 and 20 mmHg, eight (22.2%) between 20 and 25 mmHg. In two patients (5.5%), pulmonary artery pressure was > 25 mmHg, which demonstrated a tendency toward pulmonary hypertension in schistosomal portal hypertension, as previously described by our group[24].
After EGDS, we observed a significant reduction in pulmonary artery pressure in 88.2% of the patients. There was also a significant reduction in right cardiac work and right ventricular systolic work indexes, without significant increase in pulmonary vascular resistance index. The two patients without pulmonary artery pressure reduction showed a normal preoperative pulmonary artery pressure (< 15 mmHg). These findings suggest that pulmonary hyperflow may contribute to the elevated pulmonary artery pressure observed in these patients. The reduced pulmonary vascular resistance may be an accommodation of pulmonary vasculature to the hyperflow, in an attempt to maintain normal pulmonary artery pressure. Nevertheless, this adaptation seems ineffective, since pulmonary artery pressure was elevated in the majority of the patients studied. The two patients with PPHTN subjected to EGDS showed reduction of both cardiac index and mean pulmonary artery pressure.
These findings were confirmed by the hemodynamic pattern of patients subjected to DSRS, with which, 16 patients (84.2%) showed a slight increase in pulmonary artery pressure, right cardiac work and right ventricular systolic work indexes. In fact, we have previously reported the cases of two young asymptomatic patients with normal cardiovascular preoperative assessment (electrocardiography, thoracic X-ray and transthoracic echocardiography) who died 4 and 7 d after DSRS, and necropsy showed signs of acute pulmonary hypertension and right ventricular failure[25]. We hypothesized that these patients had undiagnosed preoperative elevated mean pulmonary artery pressure that caused acute pulmonary hypertension after the splenorenal shunt, due to pulmonary hyperflow. The importance of preoperative hemodynamic evaluation in hepatosplenic schistosomiasis is to identify patients with raised pulmonary artery pressure, and consequently, to choose EGDS rather than DSRS as the ideal surgical treatment.
In conclusion, the hyperdynamic circulatory state present in hepatosplenic mansonic schistosomiasis was corrected by EGDS, but it was maintained by DSRS. Similarly, the raised mean pulmonary artery pressure was corrected by EGDS and maintained by DSRS. EGDS seems to be the most physiologic surgical alternative for patients with schistosomal portal hypertension, because of its tendency to lead to immediate normalization of the systemic and pulmonary hemodynamic parameters. Cardiomyopathy present in cirrhotic portal hypertension seems to be absent in hepatosplenic mansonic schistosomiasis. Hemodynamic studies to evaluate the late circulatory pattern in these patients are necessary to confirm our findings.
To the best of our knowledge, there are no data in the literature regarding postoperative systemic hemodynamics after surgical treatment of schistosomal portal hypertension. The purpose of this study was to prospectively investigate the postoperative systemic hemodynamic effects in two different surgical procedures largely employed for treatment of schistosomal portal hypertension.
Hemodynamic changes after the surgical treatment of schistosomal portal hypertension with disconnection or shunt procedures may contribute to the understanding of the hyperdynamic circulation observed in these patients with portal hypertension and preserved liver function. This knowledge may be useful in deciding on the best surgical option for each patient.
The importance of preoperative hemodynamic evaluation in hepatosplenic schistosomiasis is to identify patients with raised pulmonary artery pressure, and consequently, choose EGDS rather than DSRS as the ideal surgical treatment. EGDS seems to be the most physiologic surgical alternative for patients with schistosomal portal hypertension, due to its tendency to lead to immediate normalization of the systemic and pulmonary hemodynamic parameters.
Schistosomal portal hypertension: presinusoidal portal hypertension in patients with preserved liver function.
This study examines in patients with pre-hepatic portal hypertension, caused by schistosomiasis, the short-term effects of two different interventions, EGDS and DSRS, on hemodynamic cardiac parameters derived from transthoracic echocardiography and direct measurement of pulmonary artery pressure. The study shows that EGDS lowers cardiac output and mean pulmonary artery pressure (probably as a result of splenectomy), while DSRS has no effect on these parameters.
S- Editor Zhu LH L- Editor Kerr C E- Editor Lu W
| 1. | Silva LC. Schistosomiasis mansoni. Clinical features. Portal hypertension: clinical and physiological aspects. Tokyo: Springer-Verlag 1991; 309-318. |
| 2. | de Cleva R, Pugliese V, Zilberstein B, Saad WA, Pinotti HW, Laudanna AA. Hyperdynamic circulation in Manson's hepatosplenic schistosomiasis. Rev Hosp Clin Fac Med Sao Paulo. 1998;53:6-10. [PubMed] |
| 3. | Cleva Rd, Saad WA, Herman P, Pugliese V, Zilberstein B, Laudanna AA, Gama-Rodrigues JJ. Portal hyperflow in patients with hepatosplenic mansonic schistosomiasis. Rev Hosp Clin Fac Med Sao Paulo. 2004;59:10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | de Cleva R, Herman P, Saad WA, Pugliese V, Zilberstein B, Rodrigues JJ, Laudanna AA. Postoperative portal vein thrombosis in patients with hepatosplenic mansonic schistosomiasis: relationship with intraoperative portal pressure and flow. A prospective study. Hepatogastroenterology. 2005;52:1529-1533. [PubMed] |
| 5. | Garcia-Tsao G. Portal hypertension. Curr Opin Gastroenterol. 2004;20:254-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Tsai MH, Iwakiri Y, Cadelina G, Sessa WC, Groszmann RJ. Mesenteric vasoconstriction triggers nitric oxide overproduction in the superior mesenteric artery of portal hypertensive rats. Gastroenterology. 2003;125:1452-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | de Cleva R, Pugliese V, Zilberstein B, Saad WA, Pinotti HW, Laudanna AA. Systemic hemodynamic changes in mansonic schistosomiasis with portal hypertension treated by azygoportal disconnection and splenectomy. Am J Gastroenterol. 1999;94:1632-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Wattanasirichaigoon S, Gordon FD, Resnick RH. Hyperdynamic circulation in portal hypertension: a comparative model of arterio-venous fistula. Med Hypotheses. 2000;55:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Møller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002;87:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 241] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | de Cleva R, Genzini T, Laudanna AA. Hipertensão portal na Esquistossomose. Revista de Medicina de São Paulo. 1996;75:126-129. |
| 11. | Ruiz-del-Arbol L, Monescillo A, Jimenéz W, Garcia-Plaza A, Arroyo V, Rodés J. Paracentesis-induced circulatory dysfunction: mechanism and effect on hepatic hemodynamics in cirrhosis. Gastroenterology. 1997;113:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 198] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Kelbaek H, Rabøl A, Brynjolf I, Eriksen J, Bonnevie O, Godtfredsen J, Munck O, Lund JO. Haemodynamic response to exercise in patients with alcoholic liver cirrhosis. Clin Physiol. 1987;7:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Benjaminov FS, Prentice M, Sniderman KW, Siu S, Liu P, Wong F. Portopulmonary hypertension in decompensated cirrhosis with refractory ascites. Gut. 2003;52:1355-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Castro M, Krowka MJ, Schroeder DR, Beck KC, Plevak DJ, Rettke SR, Cortese DA, Wiesner RH. Frequency and clinical implications of increased pulmonary artery pressures in liver transplant patients. Mayo Clin Proc. 1996;71:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 159] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Mandell MS. Critical care issues: portopulmonary hypertension. Liver Transpl. 2000;6:S36-S43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Kiely DG, Cargill RI, Struthers AD, Lipworth BJ. Cardiopulmonary effects of endothelin-1 in man. Cardiovasc Res. 1997;33:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Panos RJ, Baker SK. Mediators, cytokines, and growth factors in liver-lung interactions. Clin Chest Med. 1996;17:151-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Higenbottam T. Pathophysiology of pulmonary hypertension. A role for endothelial dysfunction. Chest. 1994;105:7S-12S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Liu H, Lee SS. Cardiopulmonary dysfunction in cirrhosis. J Gastroenterol Hepatol. 1999;14:600-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Krowka MJ. Hepatopulmonary syndrome versus portopulmonary hypertension: distinctions and dilemmas. Hepatology. 1997;25:1282-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | De BK, Majumdar D, Das D, Biswas PK, Mandal SK, Ray S, Bandopadhyay K, Das TK, Dasgupta S, Guru S. Cardiac dysfunction in portal hypertension among patients with cirrhosis and non-cirrhotic portal fibrosis. J Hepatol. 2003;39:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Hervé P, Lebrec D, Brenot F, Simonneau G, Humbert M, Sitbon O, Duroux P. Pulmonary vascular disorders in portal hypertension. Eur Respir J. 1998;11:1153-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 201] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Hadengue A, Benhayoun MK, Lebrec D, Benhamou JP. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology. 1991;100:520-528. [PubMed] |
| 24. | de Cleva R, Herman P, Pugliese V, Zilberstein B, Saad WA, Rodrigues JJ, Laudanna AA. Prevalence of pulmonary hypertension in patients with hepatosplenic Mansonic schistosomiasis--prospective study. Hepatogastroenterology. 2003;50:2028-2030. [PubMed] |
| 25. | de Cleva R, Herman P, Pugliese V, Zilberstein B, Saad WA, Gama-Rodrigues JJ. Fathal pulmonary hypertension after distal splenorenal shunt in schistosomal portal hypertension. World J Gastroenterol. 2004;10:1836-1837. [PubMed] |