Published online Oct 28, 2007. doi: 10.3748/wjg.v13.i40.5312
Revised: August 10, 2007
Accepted: August 24, 2007
Published online: October 28, 2007
AIM: To inhibit the expression of vascular endothelial growth factor (VEGF) in colon cancer cell line by RNA interference (RNAi).
METHODS: Followed the service of E-RNAi, we designed and constructed two kinds of shRNA expression vectors aiming at the VEGF gene, then transfected them into colon cancer HT29 cells by lipofectamineTM 2000. The level of VEGF mRNA was investigated by RT-PCR and Northern blotting. The protein expression of VEGF was observed by immunofluoresence staining and Western blotting.
RESULTS: We got two kinds of VEGF specific shRNA expression vectors which could efficiently inhibit the expression of VEGF in HT29 cells. RT-PCR, Northern blotting, immunofluoresence staining and Western blotting showed that inhibition rate for VEGF expression was up to 42%, 89%, 73% and 82%, respectively.
CONCLUSION: The expression of VEGF can be inhibited by RNA interference in HT29 cells.
- Citation: Li TJ, Song JN, Kang K, Tong SS, Hu ZL, He TC, Zhang BQ, Zhang CQ. RNA interference-mediated gene silencing of vascular endothelial growth factor in colon cancer cells. World J Gastroenterol 2007; 13(40): 5312-5316
- URL: https://www.wjgnet.com/1007-9327/full/v13/i40/5312.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i40.5312
Angiogenesis is a common process that is essential for tumor growth beyond 2 mm[1]. Although numerous growth factors are involved, vascular endothelial growth factor (VEGF), particularly VEGF-A, has been shown to play an important role in tumor angiogenesis[2]. VEGF, a 45 kDa
heparin-binding growth factor, is induced by hypoxia-inducible factor-1a. Binding of VEGF-A to tyrosine kinase receptors, especially VEGFR-2, mediates many key components of angiogenesis, including endothelial cell proliferation, invasion, migration, survival, as well as vessel permeability. VEGF is secreted by most rumors, including tumors of the lung, gastrointestinal tract, kidney, thyroid, bladder, ovary, and cervix, and the level of VEGF is correlated with tumor progression, invasion[3].
RNAi is the sequence-specific, posttranscriptional gene silencing method initiated by double-stranded RNAs, which are homologous to the suppressed gene. Double-stranded RNAs are processed by Dicer, a cellular RNase III, to generate duplexes of about 21nt with 3'-overhang small interfering RNA (siRNA), which mediate sequence-specific mRNA degradation. RNAi technology is not only an extremely powerful instrument for functional genomic analysis but also a potentially useful method to develop highly specific gene silencing therapeutics[4-9]. In this study, we constructed vector-based expression systems in which sense and antisense strands of short VEGF sequences were transcribed into the hairpin structure under control of the U6 promoter.
A number of studies are available on VEGF in the treatment of tumors, such as colon cancer and liver cancer[10-15]. However, due to the "off-target" of RNAi, experiments have to be done to verify the more effective sequence of VEGF before it is used in clinical practice. This study was to find a better VEGF specific RNAi for colon cancer.
RNAi vectors pShRNA-V1 and pShRNA-V2 were constructed as previously described[16-19]. In brief, 21-nucleotide-long inverted repeats (separated by a 4-nucleotide linker, ttcg) were inserted downstream of the U6 promoter. The transcribed RNA thus comprised a 21-base pair of double-stranded RNAs. Five thymidines were inserted downstream the antisense strand to provide a stop signal for the RNA polymerase. The sense strand of hairpin was homologous to a 21-nucleotide region in the target mRNA. The target sequences were selected following the advice of E-RNAi services (http://e-rnai.dkfz.de/). The sequence of V1 and V2 is 5'-TGAAGTTCATGGATGTCTATC-3' and 5'-ACATCACCATGCAGATTATGC-3', respectively. An irrelevant RNAi control plasmid was constructed for green fluorescent protein (GFP) gene, pShRNA-GFP. The sequence (5'-AGCTGACCCTGAAGTTCATCT-3') was designed to target the nucleotides 126-144 of the GFP coding region.
Human colon cancer cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 100 μg/mL streptomycin, and 100-units/mL ampicillin. The cells were plated in 24- or 6- well plates at 50%-70% confluence 24 h prior to transfection. Transfection of cells was carried out with LipofectamineTM 2000 reagent (Invitrogen, Carlsbad, CA).
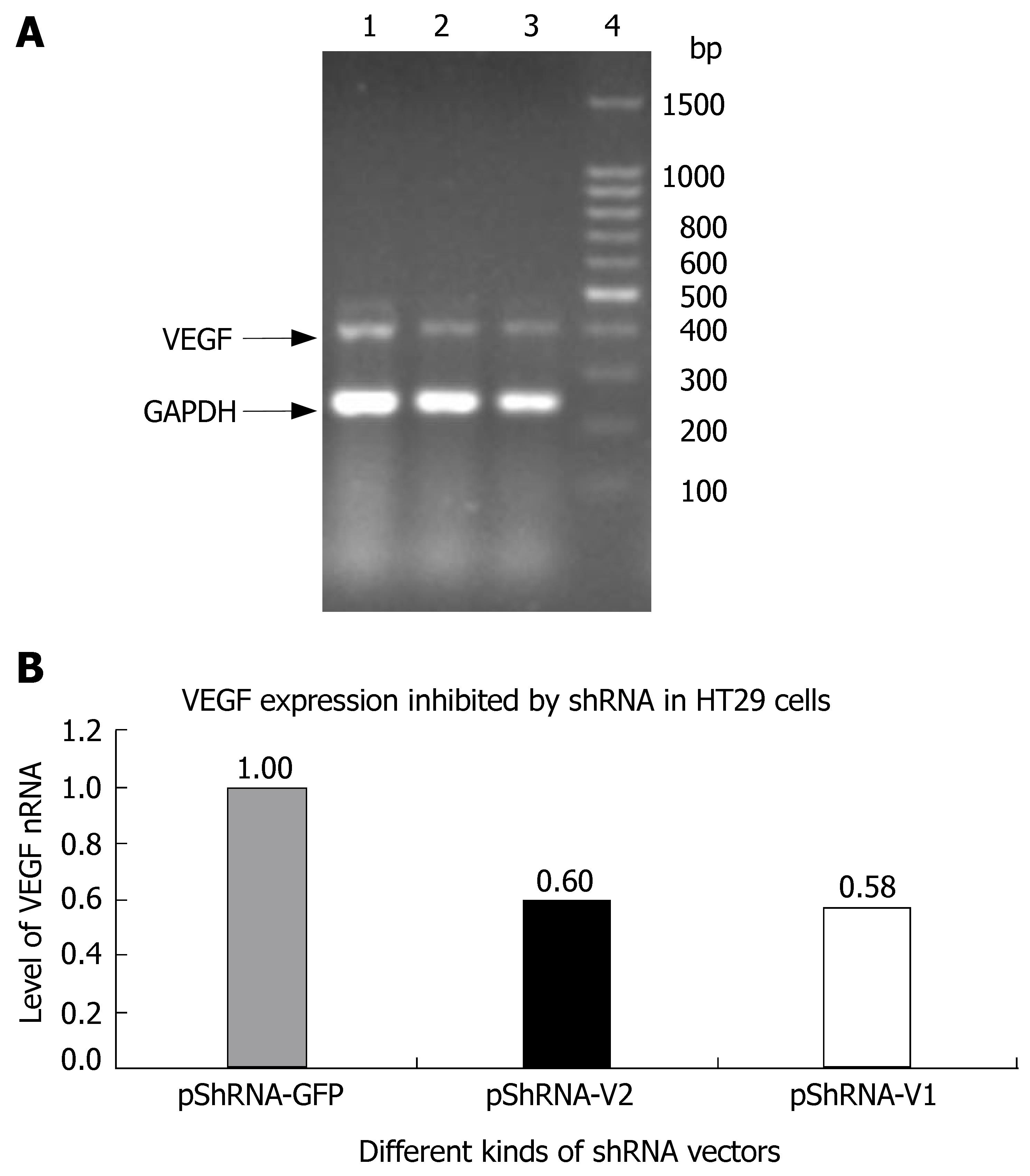
Total RNA was isolated from cultured cells and real-time polymerase chain reaction (RT-PCR) was performed using the RNeasy and one step RT-PCR kit from Qiagen Corp. RT-PCR of hGAPDH, a housekeeping gene served as a control. The sequences used for primers are 5' ctacctccaccatgccaagt-3' (sense) and 5'-aaatgctttctccgctctga-3' (antisense) for VEGF (411 bp), 5'-GGCTCTCCAGAACATCAT-3'(sense) and 5'-CACCTGGTGCTCAGTGTA-3' (antisense) for hGAPDH (240 bp). For RT-PCR, two pairs of primers were added into a reaction tube, the program consisted of an initial reverse transcription at 50°C for 30 min, denaturation at 95°C for 10 min, followed by 24 cycles of amplification (denaturation at 95°C for 30 s, annealing at 55°C for 1 min, and extension at 68°C for 1 min) and a final extension at 68°C for 10 min. The products were then separated by electrophoresis on 1.5% agarose gel, the bands were visualized using UV light and analyzed by Genetools software.
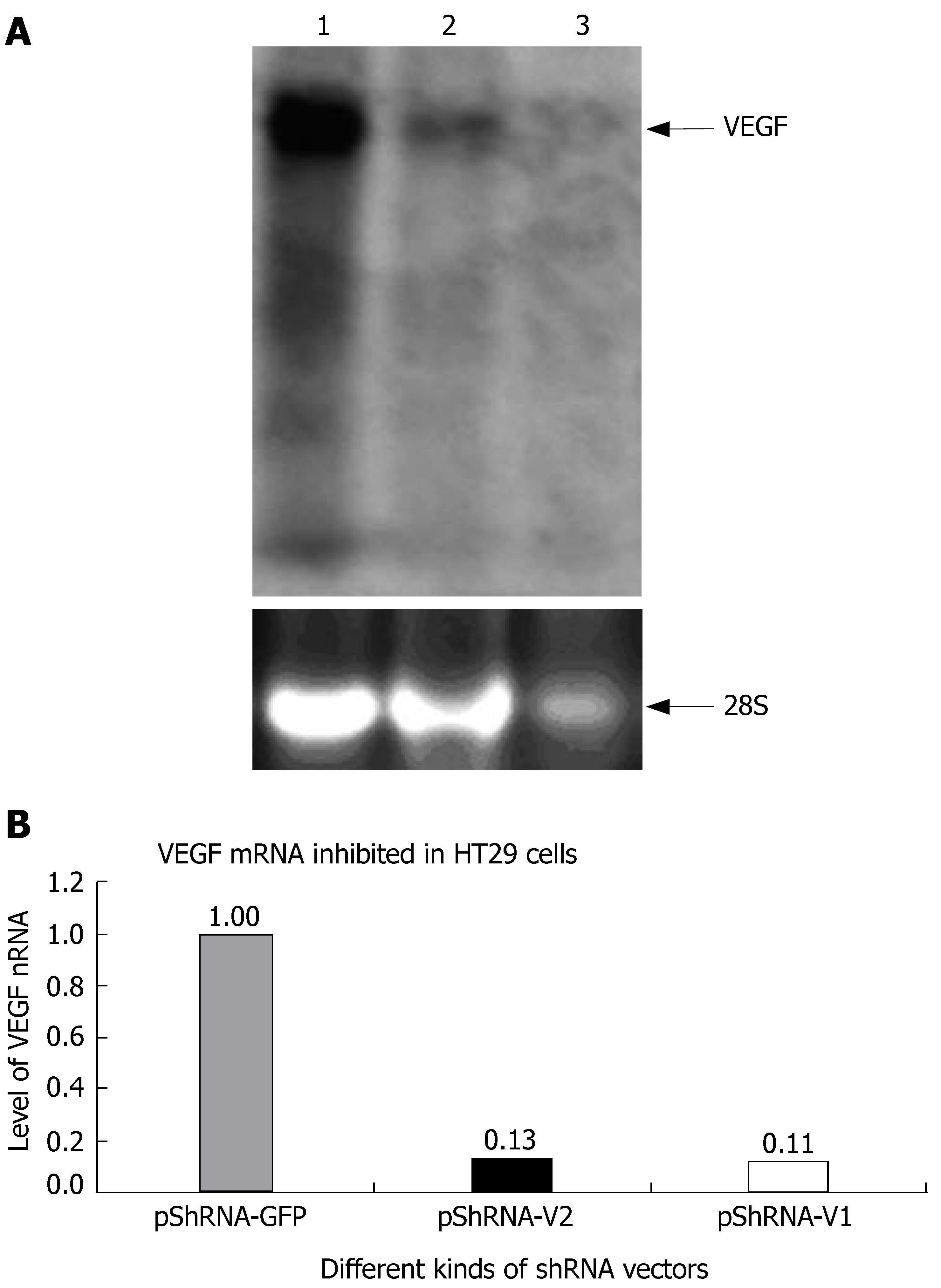
Total RNA was extracted from transfected cells on d 3 post-transfection, and purified using the RNeasy Mini Kit (Qiagen). Twenty micrograms of total RNA was separated on 1.2% agarose-formaldehyde gels and transferred onto a positively charged nylon membrane (Amersham). The presence of VEGF mRNA was probed with 32P-labeled VEGF DNA, which was generated with a random-primed labeling kit (Amersham).
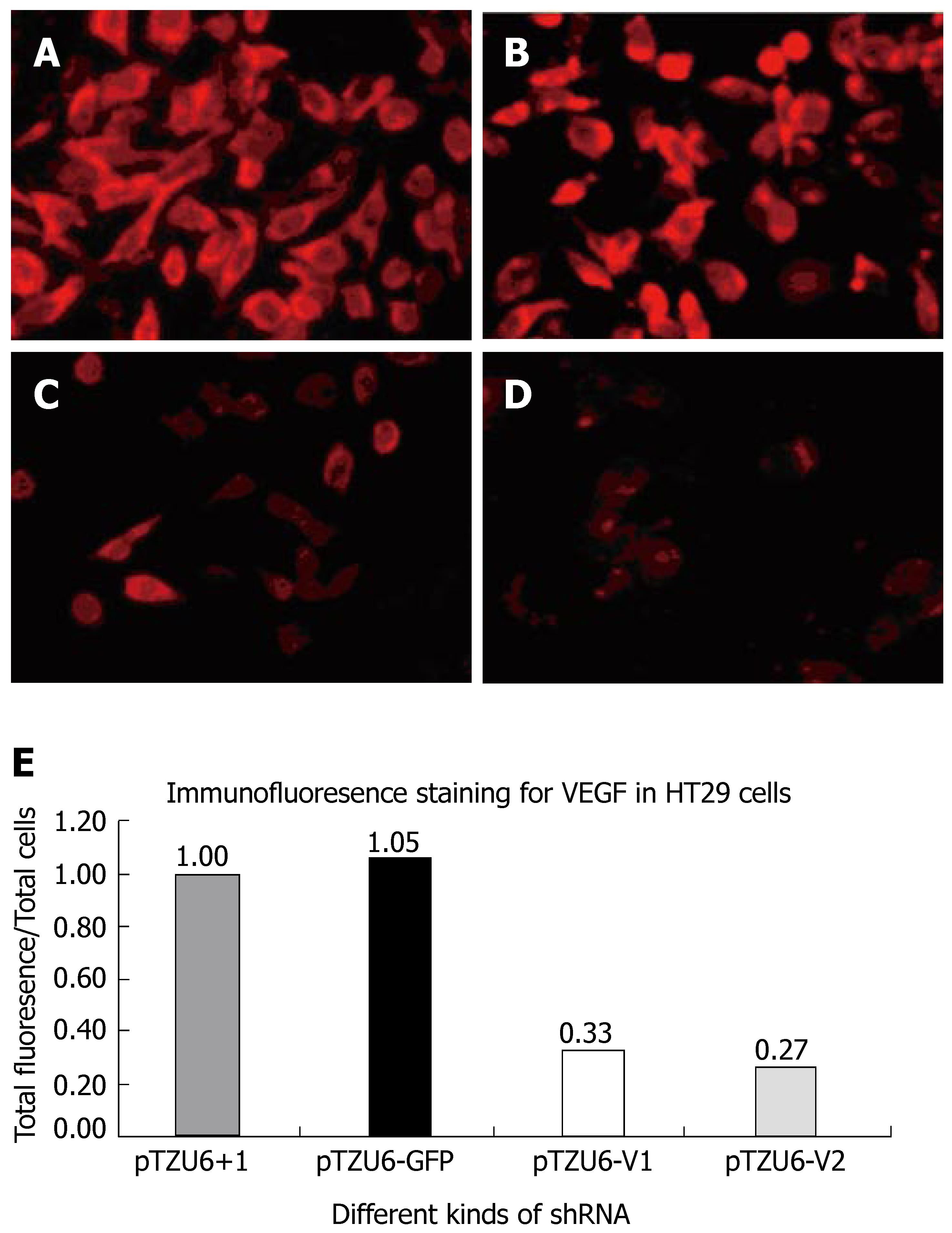
Cells were harvested on d 2 post-transfection for analysis, washed once with PBS and fixed with 4% paraformaldehyde in PBS for 20 min at 4°C. After blocked with goat serum, the cells were incubated with monoclonal mouse anti-VEGF for 2 h at 37°C. After three washes, the cells were incubated with Cy3-conjugated rabbit anti-mouse secondary antibodies for 1 h at 37°C and washed three times with PBS. The stained cells were mounted and analyzed under fluorescence microscope.
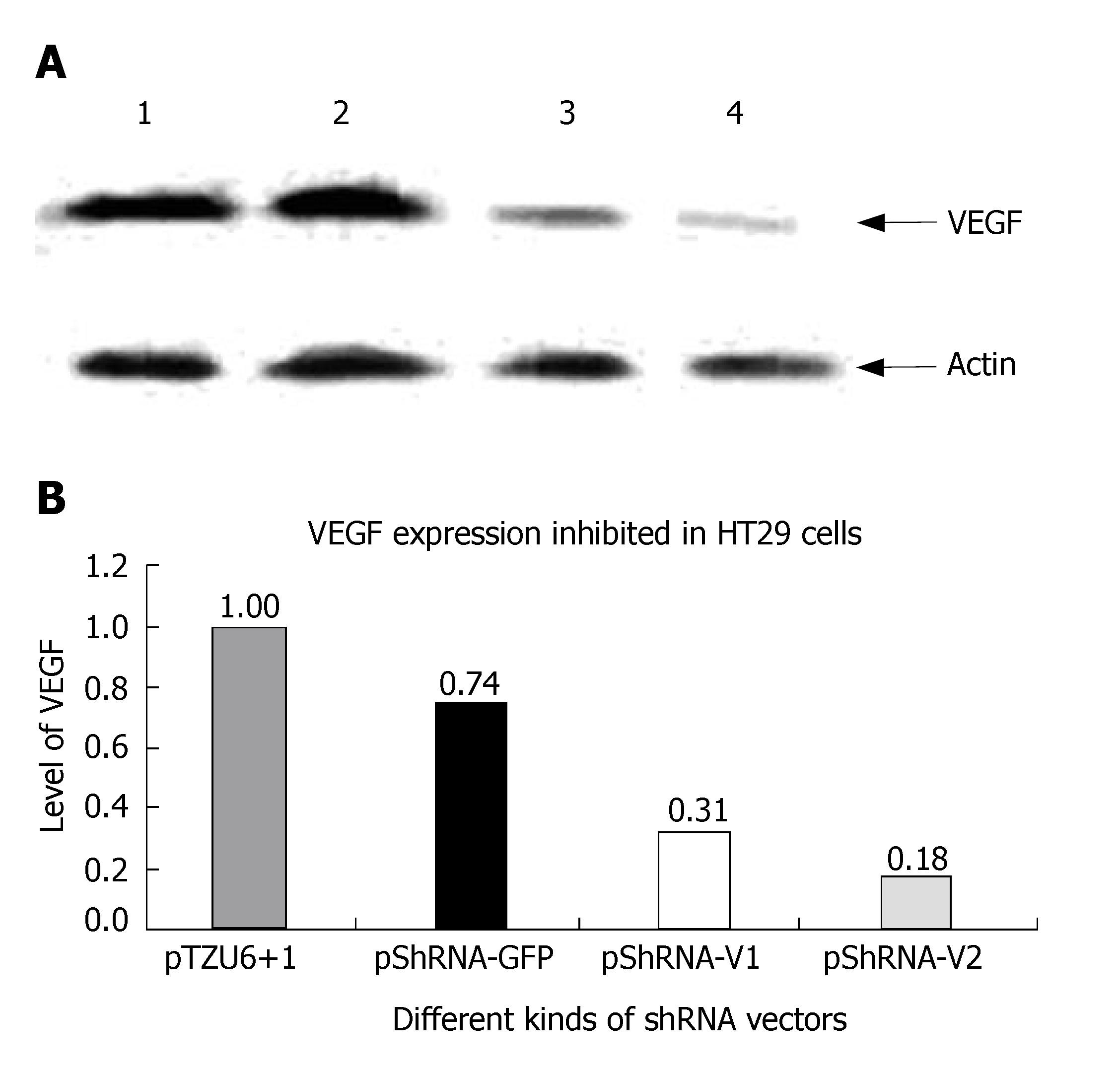
Cells were harvested on d 3 post-transfection, washed twice with 10 mL of PBS, lysed with SDS buffer, boiled for 5 min, separated by 10% SDS-PAGE gel electrophoresis, transferred onto a nitrocellulose membrane, incubated with VEGF antibodies at a dilution of 1/400 and HRP- conjugated rabbit anti-mouse antibody at a dilution of 1/4000. The HRP substrate was observed on the NC membrane. After three washes, the NC membrane was incubated with actin antibody and HRP-conjugated second antibody. The HRP substrate was observed again.
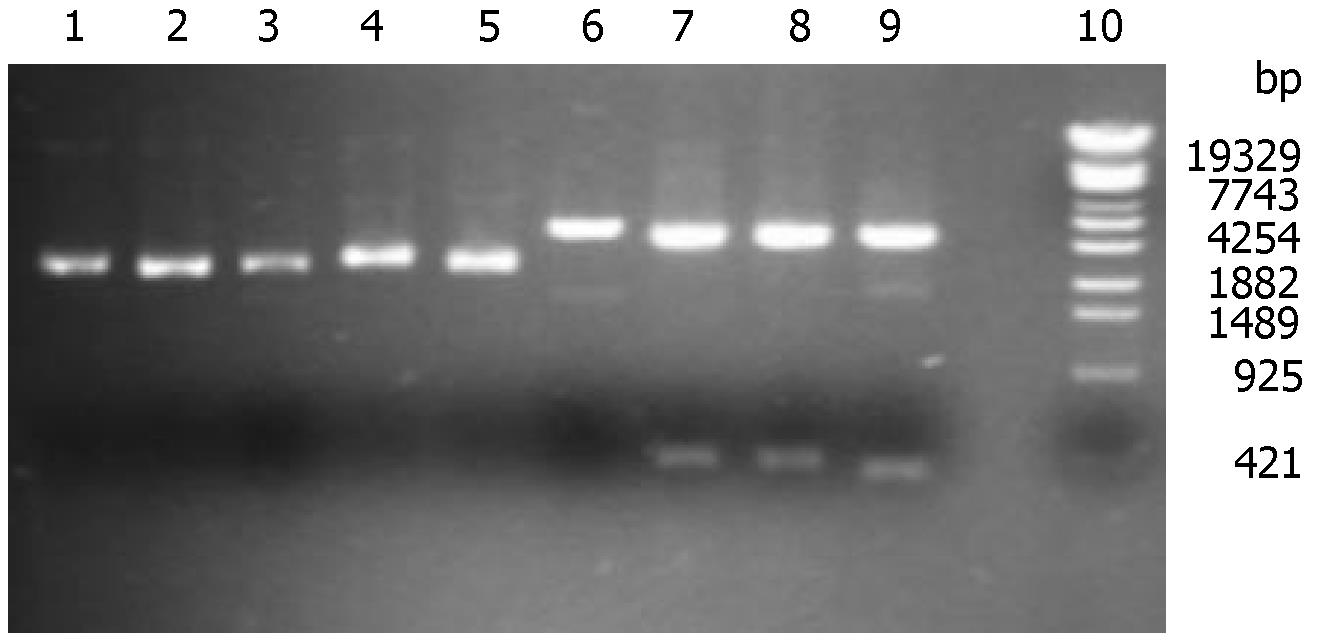
Recombinant plasmid pShRNAs could not be digested by Sal I due to the loss of Sal I site. However, the blank plasmid pTZU6+1 could be lined by sal I. When digested by Hind III and EcoR I, pShRNAs could be separated into two parts (2800 and 395 bp), and pTZU6+1 into 2800 bp and 352 bp. The correct recombinant plasmids were shown in gel electrophoresis and verified by DNA sequencing (Figure 1).
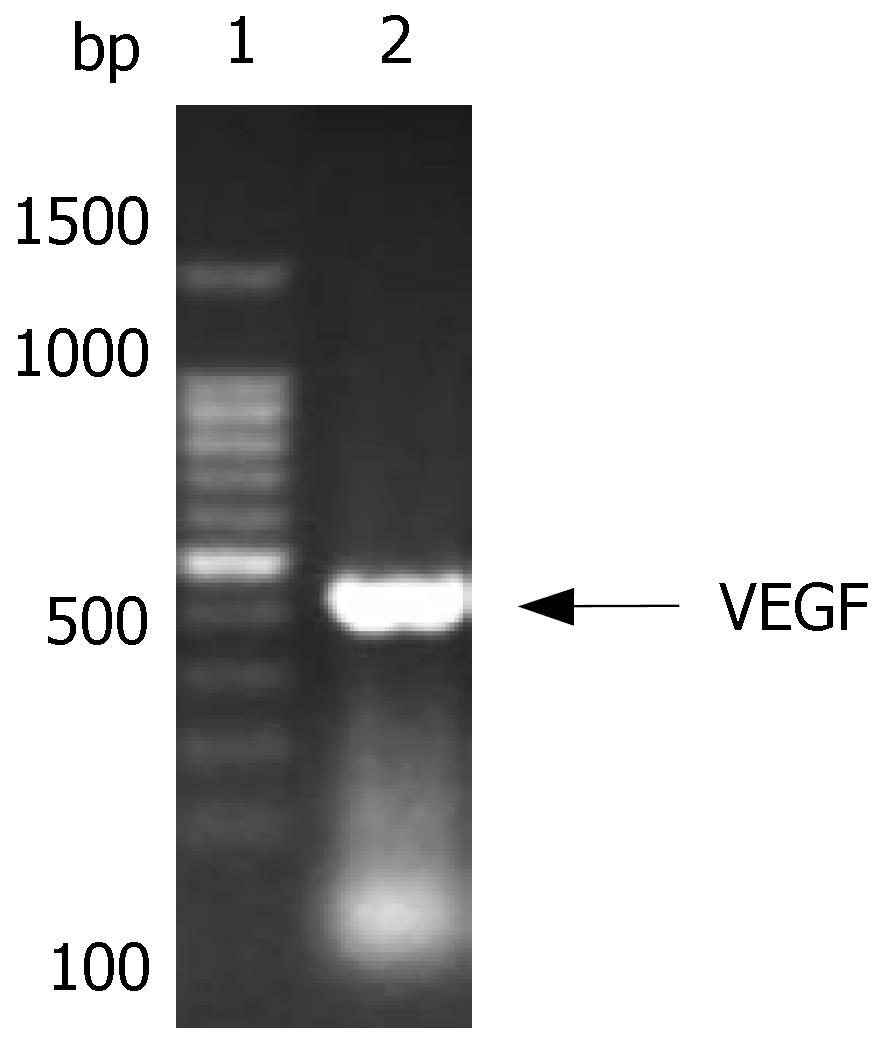
The size of VEGF was 411 bp, and consisted of the Marker in gel electrophoresis. After cloned into T vector, its sequence was verified by DNA sequencing (Figure 2).
The inhibition rate of pShRNA-V1 and pShRNA-V2 was 42% and 40% respectively in HT29 cells compared with the control plasmid pShRNA-GFP (Figure 3A and B).
The inhibition rate of pShRNA-V1 and pShRNA-V2 was 87% and 89% respectively in HT29 cells compared with the control plasmid pShRNA-GFP (Figure 4A and B).
The inhibition rate of pShRNA-V1 and pShRNA-V2 was 63% and 73% respectively in HT29 cells compared with the control plasmid pShRNA-GFP and pTZU6+1 (Figure 5A and B). VEGF was stained red and located in plasma of cells.
The inhibition rate of pShRNA-V1 and pShRNA-V2 was 69% and 82% respectively in HT29 cells compared with the control plasmid pShRNA-GFP and pTZU6+1 (Figure 6A and B).
Angiogenesis is a process of generating new capillaries from pre-existing blood vessels, which involves multiple gene products expressed by various cell types. This uncontrolled process of new blood vessel growth from the preexisting circulation network is an important pathogenic cause of tumor growth[20-24]. Although several proteins such as hepatocyte growth factor, tumor necrosis factor-α, and fibroblast growth factor 2 (FGF2) have been identified as stimulators of angiogenesis in various settings, the most important angiogenic growth factor is VEGF, which is over-expressed in many human cancers. VEGF expression in tumors can be induced by more than one mechanism. Hypoxia, which is found in most tumors, has long been known to be a potent inducer of VEGF[25-28].
In this study, shRNAs targeting VEGF efficiently reduced the transcript levels of VEGF mRNAs, and ultimately resulted in the reduction in VEGF protein levels. Furthermore, this inhibition was shown to be highly selective and sequence-specific, since control siRNAs had almost no inhibitory effect on the expression and transcription of VEGF.
There has been a considerable interest in treating a wide range of diseases with RNAi therapeutics[29,30]. In this study, in addition to the above-reported target sites in the VEGF genome, the specific 21-bp siRNAs targeting VEGF could efficiently and specifically inhibit VEGF expression, suggesting that it is a good method to inhibit the expression of VEGF.
The results of this study demonstrated that the constructed shRNA could efficiently reduce the level of VEGF transcripts and expression, suggest that shRNA-expressing vectors can be used as RNAi-based anti-VEGF therapeutics[31]. Future studies should be centered on the evaluation of the anti-VEGF efficacy of RNAi vectors in animal models, as well as on the preclinical elucidation using the RNAi technology.
The authors thank Dr. David Engelke of University of Michigan for generously providing the pTZU6+1 plasmid. This work was supported in part by research grants from the National Natural Science Foundation of China (No. 30300298) to Bing-Qiang Zhang, and the National Natural Science Foundation of China's Joint Research Fund for Overseas Chinese Young Scholars to Tong-Chuan He (No. 30228026).
Vascular endothelial growth factor (VEGF) has been shown to play an important role in tumor angiogenesis. RNAi is the sequence-specific, posttranscriptional gene silencing method initiated by double-stranded RNAs. A number of studies are available on VEGF used in the treatment of tumors, such as colon cancer, liver cancer. However, due to the "off-target" of RNAi, experiments have to be done to verify the more effective sequence of VEGF before it is used in clinical practice. This study was to find a better VEGF specific RNAi for colon cancer.
Other researches have applied VEGF in the treatment of tumors, such as colon cancer, liver cancer. There has been a considerable interest in treating a wide range of diseases with RNAi.
The results of our study suggest that shRNA-expressing vectors can be used as RNAi-based anti-VEGF therapeutics. Future studies should be centered on the evaluation of the anti-VEGF efficacy of RNAi vectors in animal models, as well as on the preclinical elucidation using the RNAi technology.
shRNA-expressing vectors can be used as RNAi-based anti-VEGF therapeutics
RNAi is the sequence-specific, posttranscriptional gene silencing method initiated by double-stranded RNAs, which are homologous to the suppressed gene. Double-strand RNAs are processed by Dicer, a cellular RNase III, to generate duplexes of about 21nt with 3'-overhang small interfering RNA (siRNA), which mediate sequence-specific mRNA degradation.
This manuscript describes the methodology for RNAi of VEGF in HT29 cells by lipofectamine. The process was evaluated quite well. No application of the technique has reported prior to this study.
Supported in part by research grants from the National Natural Science Foundation of China, No. 30300298, and the National Natural Science Foundation of China's Joint Research Fund for Overseas Chinese Young Scholars, No. 30228026
S- Editor Liu Y L- Editor Wang XL E- Editor Li HY
| 1. | Pötgens AJ, Westphal HR, de Waal RM, Ruiter DJ. The role of vascular permeability factor and basic fibroblast growth factor in tumor angiogenesis. Biol Chem Hoppe Seyler. 1995;376:57-70. [PubMed] |
| 2. | Kraizer Y, Mawasi N, Seagal J, Paizi M, Assy N, Spira G. Vascular endothelial growth factor and angiopoietin in liver regeneration. Biochem Biophys Res Commun. 2001;287:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Lu PY, Xie FY, Woodle MC. Modulation of angiogenesis with siRNA inhibitors for novel therapeutics. Trends Mol Med. 2005;11:104-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3476] [Cited by in RCA: 3275] [Article Influence: 136.5] [Reference Citation Analysis (0)] |
| 5. | Agami R. RNAi and related mechanisms and their potential use for therapy. Curr Opin Chem Biol. 2002;6:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3486] [Cited by in RCA: 3463] [Article Influence: 150.6] [Reference Citation Analysis (0)] |
| 7. | Wilda M, Fuchs U, Wössmann W, Borkhardt A. Killing of leukemic cells with a BCR/ABL fusion gene by RNA interference (RNAi). Oncogene. 2002;21:5716-5724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 195] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Cioca DP, Aoki Y, Kiyosawa K. RNA interference is a functional pathway with therapeutic potential in human myeloid leukemia cell lines. Cancer Gene Ther. 2003;10:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Aoki Y, Cioca DP, Oidaira H, Kamiya J, Kiyosawa K. RNA interference may be more potent than antisense RNA in human cancer cell lines. Clin Exp Pharmacol Physiol. 2003;30:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Mulkeen AL, Silva T, Yoo PS, Schmitz JC, Uchio E, Chu E, Cha C. Short interfering RNA-mediated gene silencing of vascular endothelial growth factor: effects on cellular proliferation in colon cancer cells. Arch Surg. 2006;141:367-374; discussion 374. [PubMed] |
| 11. | Wannenes F, Ciafré SA, Niola F, Frajese G, Farace MG. Vector-based RNA interference against vascular endothelial growth factor-A significantly limits vascularization and growth of prostate cancer in vivo. Cancer Gene Ther. 2005;12:926-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Murata M, Takanami T, Shimizu S, Kubota Y, Horiuchi S, Habano W, Ma JX, Sato S. Inhibition of ocular angiogenesis by diced small interfering RNAs (siRNAs) specific to vascular endothelial growth factor (VEGF). Curr Eye Res. 2006;31:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Fan Y, Xin XY, Chen BL, Ma X. Knockdown of RAB25 expression by RNAi inhibits growth of human epithelial ovarian cancer cells in vitro and in vivo. Pathology. 2006;38:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293:1146-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1070] [Cited by in RCA: 1050] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 15. | Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1876] [Cited by in RCA: 1803] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 16. | Paul CP, Good PD, Winer I, Engelke DR. Effective expression of small interfering RNA in human cells. Nat Biotechnol. 2002;20:505-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 578] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 17. | Sui G, Soohoo C, Affar el B, Gay F, Shi Y, Forrester WC, Shi Y. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci USA. 2002;99:5515-5520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 883] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 18. | Hannon GJ. RNA interference. Nature. 2002;418:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 2900] [Article Influence: 126.1] [Reference Citation Analysis (0)] |
| 19. | Uchida H, Tanaka T, Sasaki K, Kato K, Dehari H, Ito Y, Kobune M, Miyagishi M, Taira K, Tahara H. Adenovirus-mediated transfer of siRNA against survivin induced apoptosis and attenuated tumor cell growth in vitro and in vivo. Mol Ther. 2004;10:162-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1165] [Cited by in RCA: 1080] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 21. | McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet. 2002;3:737-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1044] [Cited by in RCA: 991] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 22. | Valdes VJ, Sampieri A, Sepulveda J, Vaca L. Using double-stranded RNA to prevent in vitro and in vivo viral infections by recombinant baculovirus. J Biol Chem. 2003;278:19317-19324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Waterhouse PM, Wang MB, Finnegan EJ. Role of short RNAs in gene silencing. Trends Plant Sci. 2001;6:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Shlomai A, Shaul Y. RNA interference--small RNAs effectively fight viral hepatitis. Liver Int. 2004;24:526-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3206] [Cited by in RCA: 3224] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 26. | Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL, Bedi A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14:34-44. [PubMed] |
| 27. | Bohula EA, Salisbury AJ, Sohail M, Playford MP, Riedemann J, Southern EM, Macaulay VM. The efficacy of small interfering RNAs targeted to the type 1 insulin-like growth factor receptor (IGF1R) is influenced by secondary structure in the IGF1R transcript. J Biol Chem. 2003;278:15991-15997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 185] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 28. | Du J, Pan Y, Shi Y, Guo C, Jin X, Sun L, Liu N, Qiao T, Fan D. Overexpression and significance of prion protein in gastric cancer and multidrug-resistant gastric carcinoma cell line SGC7901/ADR. Int J Cancer. 2005;113:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Dias S, Shmelkov SV, Lam G, Rafii S. VEGF(165) promotes survival of leukemic cells by Hsp90-mediated induction of Bcl-2 expression and apoptosis inhibition. Blood. 2002;99:2532-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 197] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 30. | Beierle EA, Strande LF, Chen MK. VEGF upregulates Bcl-2 expression and is associated with decreased apoptosis in neuroblastoma cells. J Pediatr Surg. 2002;37:467-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998;273:13313-13316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 666] [Article Influence: 24.7] [Reference Citation Analysis (0)] |