Published online Jan 28, 2007. doi: 10.3748/wjg.v13.i4.623
Revised: October 3, 2006
Accepted: December 11, 2006
Published online: January 28, 2007
AIM: To assess the therapeutic effect of Caspase-1 inhibitors (ICE-I) on acute lung injury (ALI) in experimental severe acute pancreatitis (SAP).
METHODS: Forty-two SD rats were randomly divided into 3 groups: healthy controls (HC, n = 6); SAP-S group (n = 18); SAP-ICE-I group (n = 18). SAP was induced by retrograde infusion of 5% sodium taurocholate into the bile-pancreatic duct. HC rats underwent the same surgical procedures and duct cannulation without sodium taurocholate infusion. In SAP-S group, rats received the first intraperitoneal injection of isotonic saline 2 h after induction of acute pancreatitis and a repeated injection after 12 h. In SAP-ICE-I group, the rats were firstly given ICE inhibitors intraperitoneally 2 h after induction of pancreatitis. As in SAP-S group, the injection was repeated at 12 h. Serum IL-1β was measured by ELISA. Intrapulmonary expression of Caspase-1, IL-1β and IL-18 mRNA were detected by semi-quantitative RT-PCR. The wet/dry weight ratios and histopathological changes of the lungs were also evaluated.
RESULTS: Serum IL-1β levels in SAP-S group were 276.77 ± 44.92 pg/mL at 6 h, 308.99 ± 34.95 pg/mL at 12 h, and 311.60 ± 46.51 pg/mL at 18 h, which were increased significantly (P < 0.01, vs HC). In SAP-ICE-I group, those values were decreased significantly (P < 0.01, vs SAP-S). Intrapulmonary expression of Caspase-1, IL-1β and IL-18 mRNA were observed in the HC group, while they were increased significantly in the SAP-S group (P < 0.01, vs HC). The expression of IL-1β and IL-18 mRNA were decreased significantly in the SAP-ICE-I group (P < 0.01, vs SAP-S), whereas Caspase-1 mRNA expression had no significant difference (P > 0.05). The wet/dry weight ratios of the lungs in the SAP-S group were increased significantly (P < 0.05 at 6 h, P < 0.01 at 12 h and 18 h, vs HC) and they were decreased significantly in the SAP-ICE-I group (P < 0.05, vs SAP-S). Caspase-1 inhibitors ameliorated the severity of ALI in SAP.
CONCLUSION: Caspase-1 activation, and overproduction of IL-1β and IL-18 play an important role in the course of ALI, and Caspase-1 inhibition is effective for the treatment of ALI in experimental SAP.
- Citation: Zhang XH, Zhu RM, Xu WA, Wan HJ, Lu H. Therapeutic effects of Caspase-1 inhibitors on acute lung injury in experimental severe acute pancreatitis. World J Gastroenterol 2007; 13(4): 623-627
- URL: https://www.wjgnet.com/1007-9327/full/v13/i4/623.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i4.623
Patients with severe acute pancreatitis (SAP) is often complicated with acute lung injury (ALI), which is difficult to deal with clinically. IL-1β and TNF-α are currently believed to play an important role in promoting local tissue destruction and remote organ failure in the course of SAP[1,2]. IL-18 is a novel proinflammatory cytokine, sharing striking structural and functional similarities to IL-1β. Caspase-1, also termed IL-1β-converting-enzyme (ICE), is the first member of the family of cysteine proteases called Caspases, with the functions of proteolytic cleavage of IL-1β and IL-18 precursors into their active forms. Suppression of IL-1β and IL-18 by inhibiting the function of ICE, subsequently alleviating cascade reactions, may have a therapeutic significance for SAP and systemic inflammatory response syndrome (SIRS).
In this study, an experimental model of SAP was induced in SD rats. Serum IL-1β levels, intrapulmonary expression of Caspase-1, IL-1β and IL-18 mRNA were measured respectively, and the wet/dry weight ratios and histopathological alterations in the lungs were observed to assess the therapeutic effect of Caspase-1 inhibitors on ALI in SAP.
Healthy adult male Sprague-Dawley rats weighing 230-250 g were provided by the Experimental Animal Center of Jingling Hospital in Nanjing. All forty-two rats were randomly divided into 3 groups: healthy controls (HC, n = 6); SAP-S group (n = 18); SAP-ICE-I group (n = 18). The latter two groups were further divided into 6, 12, and 18 h time points, and each contained 6 rats. SAP was induced by retrograde infusion of 5% sodium taurocholate into the bile-pancreatic duct in SD rats[3-7]. HC rats underwent the same surgical procedures and duct cannulation without sodium taurocholate infusion. In the SAP-S group, the rats received the first intraperitoneal injection of isotonic saline 2 h after induction of acute pancreatitis and a second injection after 12 h. In the SAP-ICE-I group, the rats were firstly given 0.25 mg of an ICE inhibitor (Ac-Tyr-Val-Ala-Asp-2,6-dimethylbenzoyloxymethylketone) dissolved in 1 mL sterile phosphate-buffered saline intraperitoneally 2 h after induction of pancreatitis. As in the SAP-S group, this was repeated at 12 h. Surviving rats were killed at certain time points, and all samples were obtained for subsequent analysis.
Serum IL-1β levels were measured using a commercial enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (B&C Co.). All samples were tested in duplicate and expressed as the means.
Reagents and primers: TRIZOL Reagent was purchased from Gibco BRL Life Technologies. One Step RNA PCR kit (AMV) was purchased from TaKaRa Biotechnology (Dalian) Co., Ltd. The sequences of IL-1β, IL-18 and β-actin primers (designed by Primer 3 software, synthesized by Sangon Biotechnology Co. Shanghai) were as follows: upstream and downstream primers, respectively: 5′-AAG GTC CTG AGG GCA AAG AG-3′ and 5′-GTG TTG CAG ATA ATG AGG GC-3′ for Caspase-1 (500 bp of amplification products); 5′-AGA AGC TGT GGC AGC TAC CT-3′ and 5′-TTG GGA TCC ACA CTC TCC AG-3′ for IL-1β (400 bp of amplification products); 5′-GCT GCA ATA CCA GAA GAA GG-3′ and 5′-AGA TAG GGT CAC AGC CAG TC-3′ for IL-18 (300 bp of amplification products); 5′-AGG GTG TGA TGG TGG GTA TG-3′ and 5′-CAT AGC TCT TCT CCA GGG AG-3′ for β-actin (600 bp of amplification products).
Total lung RNA extraction: Total RNA was extracted from the lung tissue by TRIZOL Reagent according to the manufacturer’s protocol. One hundred mg of lung tissue was homogenized in 1 mL of TRIZOL Reagent. Following homogenization, insoluble material was removed from the homogenate by centrifugation at 12 000 r/min for 10 min at 4°C and the homogenized tissue was incubated for 5 min at a room temperature. Then 0.2 mL of chloroform was then added. The tube was shaken vigorously for 15 s and incubated at room temperature for 3 min. The sample was centrifuged at 12 000 r/min for 15 min at 4°C and the upper aqueous phase was transferred to another tube. After that 0.5 mL of isopropyl alcohol was added. The sample was incubated at room temperature for 10 min and centrifuged at 12 000 r/min for 10 min at 4°C. The supernatant was discarded and the RNA pellets were washed with 1 mL of 75% ethanol. The samples were mixed by vortexing and centrifuged at 7000 r/min for 5 min at 4°C. At the end of the procedure, the RNA pellet was air-dried for 10 min, dissolved in 50 μL of DEPC water, and stored at -80°C. The A260/280 ratio was measured with an ultraviolet spectrophotometer and the RNA content was calculated (1A260 = 40 μg/mL).
RT-PCR was carried out using the One Step method. The total RT-PCR volume of each Eppendorf tube was 50 μL, including 5 μL of 10 × One Step RNA PCR buffer, 10 μL of MgCl2 (25 mol/L), 5 μL of dNTPs (10 mol/L), 1 μL of RNase inhibitor (40 U/μL), 1 μL of AMV RTase XL (5 U/μL), 1 μL of AMV-Optimized Taq (5 U/μL), 1 μL of upstream specific primer, 1 μL of downstream specific primer, 1 μL of experimental sample(≤ 1 μg total RNA), 24 μL of RNase Free dH2O. Each RT-PCR conditions were as follows: 30 min at 50°C for RT reactions, 2 min at 94°C for RTase inactivation, 30 s at 94°C, 30 s at 51°C, 90 s at 72°C for 30 cycles (Caspase-1); 30 s at 94°C, 30 s at 53ºC, and 90 s at 72°C for 30 cycles (IL-1β); 30 s at 94°C, 30 s at 55ºC, and 90 s at 72°C for 30 cycles(IL-18); 30 s at 94°C, 30 s at 55°C, and 90 s at 72°C for 35 cycles (β-actin). RT-PCR was terminated with an elongation step at 72°C for 5 min, and PCR products were stored at 4°C. Five μL of the reaction products was visualized by electrophoresis in 2% agarose gels containing ethidium bromide. Ultraviolet illumination was used to visualize the DNA bands, and the gels were photographed digitally. Band intensity was determined by optical density with individual PCR product/β-actin cDNA ratios.
To assess tissue edema, the right lung was removed after the experiment, then weighed and dried in a 80°C oven for 72 h until the weight was constant, and the ratio of wet weight to dry weight (W/D ratio) was then obtained.
According to routine procedures, paraffin sections of the lung tissue samples were prepared by HE staining, and histologic alterations of lung tissue were observed by light microscopy.
All values were presented as mean ± SD. Statistical analysis was performed using SPSS 11.0 statistical software applying One-Way ANOVA. A value of P < 0.05 was regarded as statistically significant.
In the SAP-S group and the SAP-ICE-I group, the serum IL-1β levels at all time points were significantly higher than those of HC (P < 0.01), whereas serum IL-1β levels were significantly decreased in the SAP-ICE-I group (P < 0.01) in comparison with the SAP-S group (Table 1).
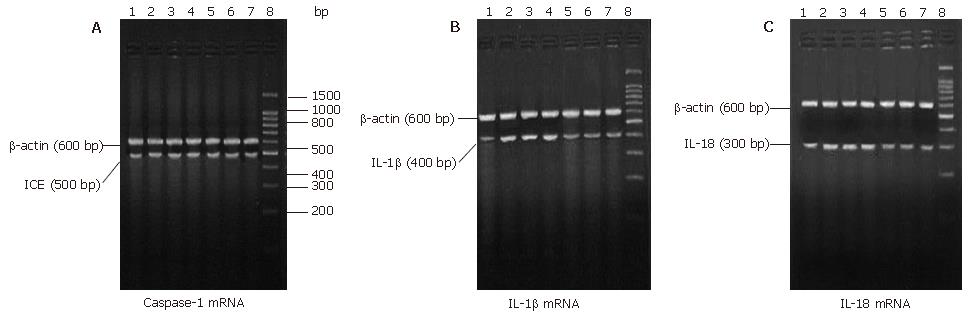
Intrapulmonary expression of Caspase-1, IL-1β and IL-18 mRNA were observed in the HC group, which were increased significantly in the SAP-S group (P < 0.01 vs HC). The expression of IL-1β and IL-18 mRNA were significantly decreased with ICE inhibition (P < 0.01), whereas Caspase-1 mRNA expression had no significant difference (P > 0.05) (Table 2, Figure 1 A-C).
| Group | n | Caspase-1 mRNA | IL-1β mRNA | IL-18 mRNA |
| HC | 6 | 0.57 ± 0.06 | 0.42 ± 0.03 | 0.55 ± 0.05 |
| SAP-S | ||||
| 6 h | 6 | 0.71 ± 0.04b | 0.75 ± 0.05b | 0.82 ± 0.05b |
| 12 h | 6 | 0.71 ± 0.05b | 0.81 ± 0.06b | 0.83 ± 0.06b |
| 18 h | 5 | 0.72 ± 0.04b | 0.79 ± 0.07b | 0.82 ± 0.07b |
| SAP-ICE-I | ||||
| 6 h | 6 | 0.72 ± 0.05b | 0.52 ± 0.05bd | 0.58 ± 0.06d |
| 12 h | 6 | 0.72 ± 0.06b | 0.50 ± 0.04bd | 0.55 ± 0.04d |
| 18 h | 6 | 0.69 ± 0.08b | 0.49 ± 0.04ad | 0.57 ± 0.04d |
In the SAP-S group, W/D ratios were increased significantly (P < 0.05 at 6 h, P < 0.01 at 12 h and 18 h, vs HC), and those were significantly attenuated with ICE inhibition (P < 0.01) (Table 1).
Observed under a light microscope, the pulmonary structures of the HC group were basically normal. However, characteristics of typical lung injury could be seen in the SAP-S group, represented by marked congestion, edema and masses of inflammatory cell infiltration in pulmonary interstitium and aveoli, thickened alveolar septum, which became progressively severe after SAP induction. Compared with the SAP-S group, such pathologic changes were much less in the SAP-ICE-I group.
SAP is often complicated with multiple systemic organ failure (MSOF), which is the major cause of death in SAP. In extra-pancreatic organs, ALI is most prominent. About 20% of cases with SAP may develop adult respiratory distress syndrome (ARDS). One third of the patients die during the early stages of SAP, a half of which die as a result of ARDS[8,9]. At present, the role of cytokines in the pathogenesis of SAP has become a hot issue in the research field. Of the numerous cytokines, IL-1β and TNF-α have been confirmed to play an important role in the development of systemic complications of SAP. However, their roles in mediating ALI during SAP are not completely understood.
Caspase-1/ICE is one member of the family of cysteine proteases called Caspases. One of the major functions of ICE is the proteolytic cleavage of the 31 000 molecular weight IL-1β precursor into its biologically active 17 000 form. IL-1β is an inflammatory cytokine produced by activated lymphocytes and monocytes. IL-1β and TNF-α are primary inducers of IL-6 and IL-8 production, and are known to cause fever, hypoperfusion, circulatory collapse, shock, metabolic acidosis, cardiac dysfunction, and the occurrence of ARDS. Norman et al[10] showed that no IL-1β mRNA expression was found in the lungs of healthy mice, and that intrapulmonary IL-1β mRNA and protein expression were increased after SAP induction. Paszkowski et al[11] demonstrated that IL-1β mRNA expression could be found in the lungs of healthy rats, and that intrapulmonary IL-1β mRNA expression was upregulated in SAP, in proportion to the severity of ALI, suggesting the existence of a significant correlation between overproduction of IL-1β in the lungs and ALI in SAP.
Ac-Tyr-Val-Ala-Asp-2,6-dimethylbenzoyloxymethylketone used in this study is a highly competitive and irreversible inhibitor of ICE. It inactivates the enzyme and is relatively inert toward other bionucleophiles such as glutathione. Previous studies showed changes in serum amylase and pancreas in SAP[12,13]. In this study, the ICE inhibitor was injected into rats intraperitoneally 2 h after SAP was induced by retrograde infusion of 5% sodium taurocholate into the bili-pancreatic duct. A remarkable elevation of serum IL-1β levels, an upregulation of intrapulmonary Caspase-1 and IL-1β mRNA expression, an increased W/D ratio of the lung and obvious pathological changes after induction of SAP were observed; serum and intrapulmonary IL-1β expression were decreased significantly, with an improvement of pathological changes in rats treated with ICE inhibitors. These suggest that the therapeutic effects of ICE inhibitors on ALI in SAP may be associated with a reduced IL-1β-mediated injury. Besides generating large numbers of active IL-1β, ICE is also known to process the inactive precursor of IL-18 into its bioactive forms[14-17]. IL-18, formerly called IFN-γ-inducing factor, is a novel proinflammatory cytokine with an 18 000 molecular weight, sharing striking structural and functional similarities to IL-1β. In addition, the biological activity of IL-18 is closely related to that of IL-1β: IL-18 induces the gene expression and synthesis of IL-1, TNF, and several chemokines by means of a putative IL-18 receptor complex. Also, IL-18 plays an important role in the Th-1 response to the stimulation of virul antigens, primarily because of its ability to induce IFN-γ production in T cells and NK cells. Rau et al[18] reported that local and systemic IL-18 concentrations are significantly elevated in patients with AP, and that serum IL-18 concentrations closely correlate with the development of pancreatic necrosis and remote organ failure. In the current study, intrapulmonary IL-18 mRNA expression was measured by RT-PCR. Marked upregulation of IL-18 mRNA was observed in the lungs after induction of SAP, and intrapulmonary IL-18 expression was significantly decreased in rats treated with ICE inhibitors. In addition, it is well established that IL-1β, IL-18, and TNF-α share a close interrelationship by inducing the synthesis of each other[14,19,20]. Paszkowski et al[11] indicated that intrapulmonary TNF-α mRNA expression was uniformly downregulated in rats receiving the treatment of ICE inhibitors, suggesting that the therapeutic effects of ICE inhibitors on ALI in SAP may correlate with a decrease in TNF-α-mediated injury. Considering that the synthesis of IL-1β, IL-18, and TNF-α could be induced by each other, plus the alterations in W/D ratios of the lungs and histopathologic changes in SAP, we speculate that as with TNF-α and IL-1β, overproduction of IL-18 in the lungs plays an important role in the course of SAP complicated with ALI, and that therapeutic effects of ICE inhibitors on ALI in SAP may be associated with the inhibition of IL-18.
In summary, activation of Caspase-1/ICE, and overproduction of IL-1β and IL-18 in the lungs play an important role during the course of ALI and ARDS in SAP, and ICE inhibitors are effective against ALI in SAP. The mechanisms for ICE inhibition may be associated with decreased cytokine-mediated injury, such as IL-1β, IL-18 and TNF-α. Therefore, studies of the mechanism for ALI in SAP may shed new light on understanding of SIRS and MSOF, as well as the prevention and treatment for SAP.
We thank Professor Zhao-Shen Li and Zhen-Xing Tu, Department of Gastroenterology, Changhai Hospital, Shanghai, for their expert assistance in this study.
S- Editor Liu Y L- Editor Zhu LH E- Editor Lu W
| 1. | Laveda R, Martinez J, Munoz C, Penalva JC, Saez J, Belda G, Navarro S, Feu F, Mas A, Palazon JM. Different profile of cytokine synthesis according to the severity of acute pancreatitis. World J Gastroenterol. 2005;11:5309-5313. [PubMed] |
| 2. | Granger J, Remick D. Acute pancreatitis: models, markers, and mediators. Shock. 2005;24 Suppl 1:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 180] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 3. | Aho HJ, Koskensalo SM, Nevalainen TJ. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 258] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Lange JF, van Gool J, Tytgat GN. Experimental pancreatitis in the rat: role of bile reflux in sodium taurocholate-induced acute haemorrhagic pancreatitis. Eur Surg Res. 1986;18:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Rau B, Paszkowski A, Lillich S, Baumgart K, Möller P, Beger HG. Differential effects of caspase-1/interleukin-1beta-converting enzyme on acinar cell necrosis and apoptosis in severe acute experimental pancreatitis. Lab Invest. 2001;81:1001-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Nakamura H, Honda H, Tashiro M, Taguchi M, Yoshikawa H, Otsuki M. Increased expression of 19-kD interacting protein-3-like protein and the relationship to apoptosis in the lung of rats with severe acute pancreatitis. Crit Care Med. 2003;31:2527-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Meng Y, Zhang M, Xu J, Liu XM, Ma QY. Effect of resveratrol on microcirculation disorder and lung injury following severe acute pancreatitis in rats. World J Gastroenterol. 2005;11:433-435. [PubMed] |
| 8. | Steer ML. Relationship between pancreatitis and lung diseases. Respir Physiol. 2001;128:13-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Bhatia M, Slavin J, Cao Y, Basbaum AI, Neoptolemos JP. Preprotachykinin-A gene deletion protects mice against acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2003;284:G830-G836. [PubMed] |
| 10. | Norman JG, Fink GW, Denham W, Yang J, Carter G, Sexton C, Falkner J, Gower WR, Franz MG. Tissue-specific cytokine production during experimental acute pancreatitis. A probable mechanism for distant organ dysfunction. Dig Dis Sci. 1997;42:1783-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 174] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Paszkowski AS, Rau B, Mayer JM, Möller P, Beger HG. Therapeutic application of caspase 1/interleukin-1beta-converting enzyme inhibitor decreases the death rate in severe acute experimental pancreatitis. Ann Surg. 2002;235:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Liu HB, Cui NQ, Li DH, Chen C. Role of Kupffer cells in acute hemorrhagic necrotizing pancreatitis-associated lung injury of rats. World J Gastroenterol. 2006;12:403-407. [PubMed] |
| 13. | Mota RA, Sánchez-Bueno F, Saenz L, Hernández-Espinosa D, Jimeno J, Tornel PL, Martínez-Torrano A, Ramírez P, Parrilla P, Yélamos J. Inhibition of poly(ADP-ribose) polymerase attenuates the severity of acute pancreatitis and associated lung injury. Lab Invest. 2005;85:1250-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci. 1998;856:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 390] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 15. | Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1). J Clin Immunol. 1999;19:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 372] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 16. | Cordoba-Rodriguez R, Fang H, Lankford CS, Frucht DM. Anthrax lethal toxin rapidly activates caspase-1/ICE and induces extracellular release of interleukin (IL)-1beta and IL-18. J Biol Chem. 2004;279:20563-20566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Coward WR, Marei A, Yang A, Vasa-Nicotera MM, Chow SC. Statin-induced proinflammatory response in mitogen-activated peripheral blood mononuclear cells through the activation of caspase-1 and IL-18 secretion in monocytes. J Immunol. 2006;176:5284-5292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Rau B, Baumgart K, Paszkowski AS, Mayer JM, Beger HG. Clinical relevance of caspase-1 activated cytokines in acute pancreatitis: high correlation of serum interleukin-18 with pancreatic necrosis and systemic complications. Crit Care Med. 2001;29:1556-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 510] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 20. | Ogawa M. Acute pancreatitis and cytokines: "second attack" by septic complication leads to organ failure. Pancreas. 1998;16:312-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 3.3] [Reference Citation Analysis (0)] |