Published online Oct 21, 2007. doi: 10.3748/wjg.v13.i39.5267
Revised: July 1, 2007
Accepted: August 24, 2007
Published online: October 21, 2007
AIM: To evaluate the relationship between changes in serum transforming growth factor β1 (TGFβ1) level and curative effect of radiotherapy (RT) in patients with esophageal carcinoma.
METHODS: Ninety patients with histologically confirmed esophageal carcinoma were enrolled. Serum samples for TGFβ1 analysis were obtained before and at the end of RT. An enzyme-linked immunosorbent assay was used to measure serum TGFβ1 level. Multivariate analysis was performed to investigate the relationship between disease status and changes in serum TGFβ1 level.
RESULTS: Serum TGFβ1 level in patients with esophageal carcinoma before RT was significantly higher than that in healthy controls (P < 0.001). At the end of RT, serum TGFβ1 level was decreased in 67.82% (59/87) of the patients. The overall survival rate at 1, 3 and 5 years was 48.28% (42/87), 19.54% (17/87) and 12.64% (11/87), respectively. Main causes of death were local failure and regional lymph node metastasis. In patients whose serum TGFβ1 level decreased after RT, the survival rate at 1, 3 and 5 years was 61.02% (36/59), 28.81% (17/59) and 18.64% (11/59), respectively. The survival rate at 1 year was 17.86% (5/28) in patients whose serum TGFβ1 level increased after RT, and all died within 18 mo (P < 0.01).
CONCLUSION: Serum TGFβ1 level may be a useful marker for monitoring disease status after RT in patients with esophageal carcinoma.
- Citation: Sun SP, Jin YN, Yang HP, Wei Y, Dong Z. Serum transforming growth factor-β1 level reflects disease status in patients with esophageal carcinoma after radiotherapy. World J Gastroenterol 2007; 13(39): 5267-5272
- URL: https://www.wjgnet.com/1007-9327/full/v13/i39/5267.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i39.5267
Esophageal carcinoma is one of the most common malignant diseases in China. However, for a large number of patients, treatment is only palliative. The 5-year survival rate has remained about 10% for patients treated with conventionally fractionated radiotherapy (CR) alone. The poor prognosis is the result of both loco-regional treatment failure, seen in up to 80% of cases, and early disease dissemination[1,2]. Failure of local control remains a significant clinical problem. Therefore, local control to improve survival of patients with esophageal carcinoma patients has been focused on by most investigators. Much emphasis has been placed on the role of physical factors (e.g., total dose and dose per fraction) in improving local control and survival rate. In China, investigators have published their results on esophageal carcinoma, using a schedule named late-course hyperfractionation accelerated radiation therapy (LCHART)[3-7]. In this way, the survival rate of patients with esophageal carcinoma has been increased from 10% to 30%. However, acute toxicity reactions, mainly esophagitis, have increased. Although physical factors are important, none of these models considers the molecular biological events that may be responsible for the observed heterogeneity in tumor tissue response.
Cytokines play a key role in regulation of cells of the immune system and have also been implicated in the pathogenesis of malignant diseases. Transforming growth factor beta-1 (TGFβ1) is a cytokine with multiple biological functions. It influences the proliferation rate of many cell types, and acts as a growth inhibitor in most but not all cases. In addition, TGFβ1 controls the process of epithelial cell differentiation. In normal cells, TGFβ1 generally enhances adhesion through increased cell matrix production and decreased prote-olysis. Resistance to the negative growth-regulating properties of TGFβ1 has been observed in epithelial and mesenchymal tumors. In addition to acting as a stimulator of angiogenesis, TGFβ1 also influences the growth of tumor cells directly or indirectly. Tumor cells can escape the inhibitory effects of TGFβ1 on normal cells at the post-transcription, receptor or post-receptor level. When tumor cells are insensitive, TGFβ1 can also promote tumor metastasis by enhancing angiogenesis, and adjusting the character of the matrix, or adjusting the body's immune response to tumor growth. Animal experiments and clinical observations have demons-trated the functions of TGFβ1 in radiation-induced injury of normal tissues[8,9]. It has been implicated in the injury of several organs after irradiation, including the lungs and breasts, especially in radiation-induced pneumonitis (RP)[10-13]. It also has been proposed that serial measure-ments of plasma TGFβ1 can be valuable for estimating the risk of RP and deciding whether additional dose-escalation can be safely applied. In recent studies, a relationship between prognosis of many tumors and this cytokine has also been found[14-17]. These suggest that TGFβ1 may be a promising prognostic marker for some cancer patients. However, Fukai's data have suggested that an elevated systemic TGFβ1 level is not related to tumor progression in esophageal cancer[18]. For this reason, they think that systemic inflammation or chronic disease, in addition to the tumor itself, may influence plasma TGFβ1 level. However, another study has shown a significant correlation between TGFβ1 level measured in the azygos vein and distant lymph node metastasis in esophageal cancer[19]. Based on these findings, we hypothesized that serial blood TGFβ1 measurements can be used to identify disease status in patients with esophageal carcinoma treated with conventional doses of radiotherapy (RT), and as a potential predictive marker that may allow us to stratify patients into different treat-ment groups. Here, we detected serum TGFβ1 level in 90 patients with esophageal carcinoma before and at the end of RT, to investigate the relationship between changes in serum TGFβ1 level and disease status in patients with esophageal carcinoma after RT.
From August 1997 to June 1998, 90 unresectable or medically inoperable patients were enrolled into our clinical trial. Only patients with histologically confirmed esophageal carcinoma were eligible. The additional criteria for eligibility were age ≤ 75 years, Karnofsky performance status ≥ 70, white blood cell and hemoglobin levels within the normal range, and no prior treatment. The patients' clinical characteristics are listed in Table 1. The pretreatment evaluation generally included medical history and physical examination, complete blood cell count, chest radiography and/or chest computed tomography (CT), esophageal barium examination, and ultrasound examination of the abdomen, including the liver, kidneys, spleen and retroperitoneal lymph nodes. Based on the exa-minations mentioned above, patients were staged according to the TNM staging system of the 1997 American Joint Committee on Cancer staging system.
| Characteristic | Number of patients |
| Gender | |
| Male | 64 |
| Female | 26 |
| Age (yr) | |
| Range | 42-75 |
| Median | 57.80 |
| Pathology | |
| Squamous cell carcinoma | 88 |
| Undifferentiated carcinoma | 2 |
| Location | |
| Upper-thoracic | 37 |
| Middle-thoracic | 41 |
| Lower-thoracic | 12 |
| Length (cm) | |
| Median | 5.91 |
| Range | 2-12 |
| Stage (UICC 1997) | |
| I | 8 |
| IIa | 31 |
| IIb | 45 |
| III | 6 |
The study, including the criteria for patient eligibility, diagnostic procedures, fractionation schemes for treatment techniques, collection of blood samples, and tests, was approved by the Ethical Committee of Changhai Hospital. All patients received full information concerning the aim of the study, diagnostic and treatment procedures, medical care, and risks of acute and late sequelae before they entered the trial, and all patients voluntarily gave informed consent.
All patients were given RT alone. A 10 MV X-ray linear accelerator was used for treatment. The design of the radiation fields was based on the diagnosis by CT and barium examination. For all patients, a three-field approach was administered: one anterior and two posterior oblique portals. The width of the fields was adjusted to cover gross tumors with 2-3 cm extended margins, so as to include subclinical lesions. The length of the field covered clinical tumors with a 3-5 cm extended margin at both ends of the lesion. All patients received conventional fractions, 2.0 Gy per fraction, five fractions per week. The total dose given to the tumor was 60-70 Gy/6-7 W. Lung corrections were not performed in this study.
At the end of RT, all patients received esophageal barium examination and the clinical radiation response was evaluated according to standard X-ray diagnosis of eso-phageal carcinoma after RT[5]. A complete response (CR) was the disappearance of the mass shadow, no narrowing observed in the esophageal lumen, and none or slight rigidity of the esophageal wall without residual ulceration. Partial response (PR) was > 50% reduction in tumor bulk, but < 100% resolution of the disease and a residual shallow ulcer with a diameter < 1.5 cm, despite the disappearance of the mass shadow. Minor response (MR) was definite improvement in the barium esophagogram, but with < 50% regression, with a large residual ulcer crater and/or narrowing of the esophageal lumen, re-gardless of the residual state of the mass shadow. No change (NC) was no improvement in the X-ray findings, with a deep and large residual ulcer or complete obstru-ction of the esophageal lumen, regardless of the residual state of the mass shadow.
The main endpoint in this analysis was the relationship between survival rate and change in serum TGFβ1 level. Death from any cause was calculated from the starting date of RT until death or the last follow-up evaluation. After treatment, follow-up included medical oncology visits at 3-mo intervals for 1 year, and then every 6 mo thereafter up to 5 years. The relationship between 1, 3 and 5-year survival rates and change in serum TGFβ1 level was observed.
Blood samples (2 mL each) were collected in EDTA tubes and stored for 1-3 h at 4°C, until the samples were centrifuged for plasma removal. Blood samples were centrifuged at 2000 g for 20 min, and only the top 0.5-1.0 mL plasma supernatant (serum) was removed to avoid platelet contamination. The serum samples were kept frozen at -70°C until assayed for TGFβ1. An enzyme-linked immunosorbent assay (ELISA) was used to determine TGFβ1 level. The TGFβ1 ELISA kit was purchased from R&D Systems (Shanghai, China). Serum samples were not subjected to acid/ethanol extraction, and active TGFβ1 was measured using the kit according to the manufacturer's recommended procedures. The control population consisted of 15 samples from normal blood donors.
The Statistical Package for Social Sciences, version 10.0. was used for statistical analysis. Serum TGFβ1 levels were expressed as means ± SD. Two sample means were statistically compared using Student's t test, assuming an unequal variance. Multiple comparisons between the mean TGFβ1 concentrations were performed using analysis of variance. The Kaplan-Meier model was used to estimate survival, and the differences between them were compared by the log-rank test.
All patients were followed until death or the time of analysis. Three patients were interrupted during RT and were removed from the statistical analysis, and five patients were lost to follow-up, who were counted as being dead at the time they disappeared. Eighty-two patients were followed for 5 years and the follow-up rate was 94.25%.
As shown in Table 2, before RT, mean serum TGFβ1 level in patients with esophageal cancer was 41.13 ± 15.41 ng/mL. This concentration was significantly higher than that in controls (9.53 ± 6.45 ng/mL) (P < 0.001). At the end of RT, a decreased serum TGFβ1 level was found in 67.82% (59/87) of all patients that completed the schedule. This suggested that TGFβ1 in the blood was produced by the esophageal tumor, and that its decrease may be due to the tumors being controlled by RT.
| Groups | n | Mean value (ng/mL) | t value | P value |
| Control | 15 | 9.53 ± 6.45 | 5.287 | 0.001 |
| All patients | ||||
| Before RT | 90 | 41.13 ± 15.41 | ||
| After RT | 87 | 36.52 ± 19.26 | 1.365 | 0.072 |
| Decreased group | ||||
| Before RT | 59 | 42.93 ± 14.37 | ||
| After RT | 59 | 25.98 ± 8.39 | 2.481 | 0.006 |
| Increased group | ||||
| Before RT | 28 | 40.25 ± 16.29 | ||
| After RT | 28 | 51.61 ± 19.75 | 1.827 | 0.039 |
The relationship between clinical response to RT and serum TGFβ1 level is listed in Table 3. In patients with a reduced TGFβ1 level, tumor response rate to RT (CR plus PR) was 83.05% (49/59), and it was 67.86% (19/28) in those showing an increase (P < 0.05).
| TGFβ1 | n | CR | PR | MR | NC |
| Decrease | 59 | 32.20 (19/59) | 50.85 (30/59) | 13.56 (8/59) | 3.39 (2/59) |
| Increase | 28 | 21.43 (6/28) | 46.43 (13/28) | 25.00 (7/28) | 7.14 (2/28) |
It was found in stage III patients that serum TGFβ1 level was significantly decreased at the end of RT compared to that before RT. Mean serum TGFβ1 levels before and after therapy in these patients were 42.38 ± 13.65 and 34.76 ± 15.62, respectively (P < 0.05). Three months after RT, 11.5% (10/87) of patients had local treatment failure and regional lymph node metastasis. Among these patients, nine out of 28 (32%) patients' serum TGFβ1 levels increased, while only one out of 59 (1.7%) patients had a decreased TGFβ1 level. Comparatively, the difference between change in serum TGFβ1 level and local treatment failure and metastasis was significant (P < 0.001). The patterns of failure and metastasis are listed in Table 4.
| Case | Location | Stage | Response to RT | Failure or metastasis | TGFβ1 (ng/mL) | |
| Before | After | |||||
| 1 | Upper | III | NC | Lymph nodes M | 30.10 | 42.80 |
| 2 | Middle | IIb | NC | Lymph nodes M | 51.95 | 67.65 |
| 3 | Upper | IIa | PR | Lymph nodes M | 18.60 | 38.20 |
| 4 | Upper | III | PR | Lymph nodes and bone M | 25.93 | 28.73 |
| 5 | Middle | III | PR | Trachea M | 29.45 | 29.55 |
| 6 | Lower | IIa | PR | Regional failure | 23.05 | 27.18 |
| 7 | Middle | IIa | PR | Regional failure | 40.68 | 19.88 |
| 8 | Middle | IIb | PR | Regional failure | 30.08 | 62.65 |
| 9 | Upper | I | CR | Regional failure | 40.73 | 49.03 |
| 10 | Upper | I | PR | Lymph nodes M | 13.00 | 32.65 |
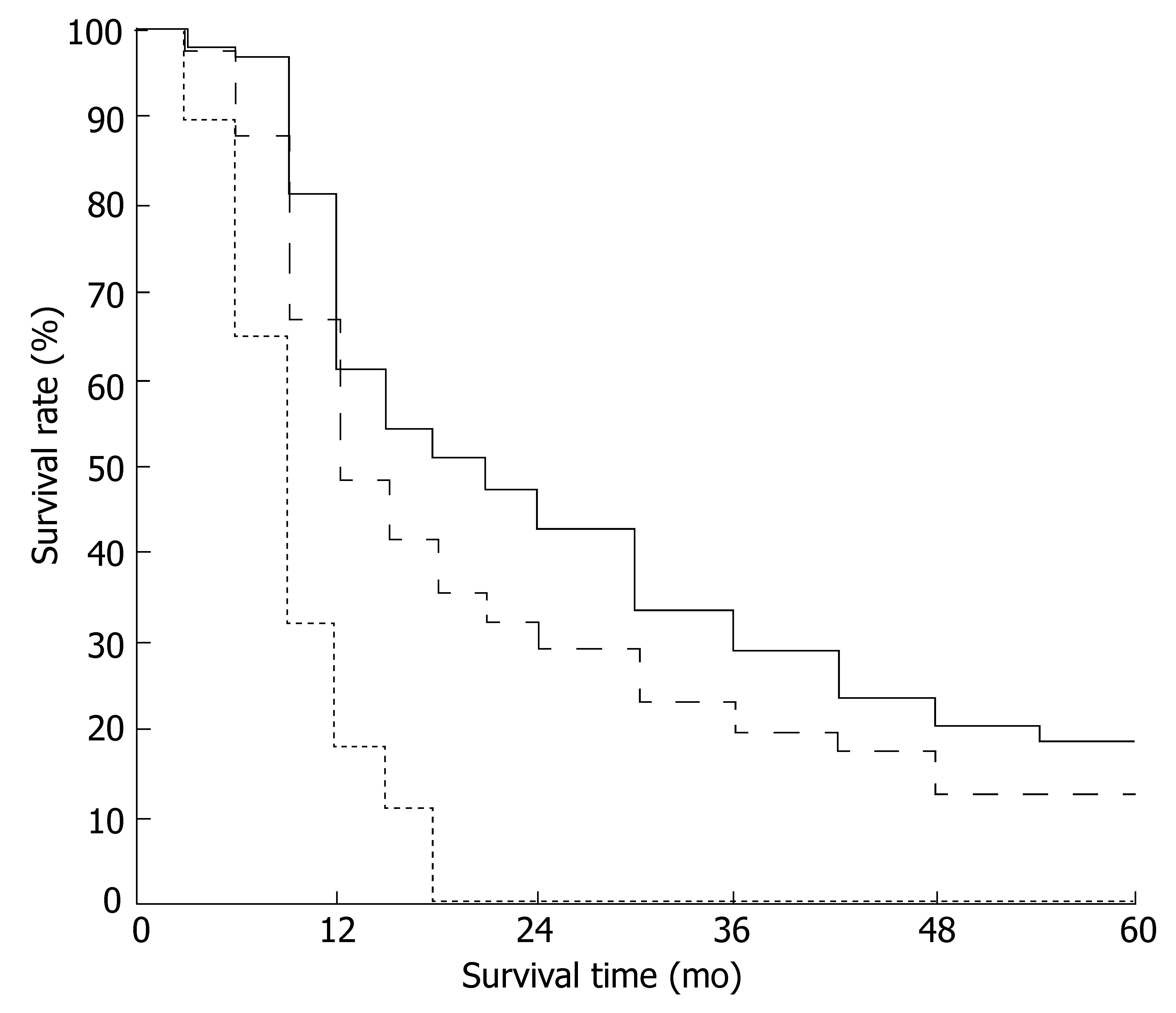
Five patients were lost to follow-up between 1 and 5 years after RT, and were counted as deaths from the day that they died. The overall survival rate at 1, 3 and 5 years was 48.28% (42/87), 19.54% (17/87) and 12.64% (11/87), respectively. Main causes of death were local treatment failure and regional lymph node metastasis. In patients with decreased serum TGFβ1 level after RT, survival rate at 1, 3 and 5 years was 61.02% (36/59), 28.81% (17/59) and 18.64% (11/59), respectively. The survival rate at 1 year was 17.86% (5/28) in patients with increased serum TGFβ1 levels after RT, and all died within 18 months (P < 0.01). The survival curves for patients in the three groups are shown in Figure 1.
In the present study, we investigated the relationship between changes in serum TGFβ1 level and curative effect of RT in patients with esophageal carcinoma, and the function of the cytokine TGFβ1 in treatment prognosis or disease status after RT. Data showed that average serum TGFβ1 level in patients with esophageal carcinoma before RT was significantly higher than that of healthy controls, which suggests that TGFβ1 in the blood is produced by esophageal tumors. After RT, serum TGFβ1 level was reduced in 67.82% (59/87) of patients compared to that before RT. This may be because the tumors were controlled by RT. This is believed to be the first report that there is a relationship between serum TGFβ1 level and esophageal carcinoma.
As mentioned above, much emphasis has been placed on the role of physical factors (e.g., total dose and dose per fraction) in improving local control and survival rate of esophageal carcinoma. Although physical factors are important, molecular biological events may be responsible for the observed heterogeneity in tumor tissue response between patients.
Recently, investigators have shown that changes in blood levels of certain cytokines, such as TGFβ1, may predict the risk of radiation-induced lung injury and association with disease progression[20-22]. In a study of 73 patients receiving high-dose thoracic RT for lung cancer, Anscher et al[23] found that those patients whose plasma TGFβ1 level was normal at the completion of RT were at low risk for subsequent radiation-induced lung injury, whereas the risk of symptomatic lung damage was increased in patients whose TGFβ1 level remained elevated. Subsequent analysis showed that these changes in plasma TGFβ1 correlated with the risk of pulmonary injury, independent of the volume of lung irradiated. TGFβ1 seems to affect tumor angiogenesis and play an important role in tumor progression in non-small cell lung carcinoma. Kong et al[24] measured plasma TGFβ1 concentrations before, during and after RT in 54 patients with lung cancer non-small cell lung cancer (NSCLC), to determine the kinetics of TGFβ1 expression during and after RT, and to correlate plasma TGFβ1 level with disease status after treatment. The results show that in those patients with an elevated plasma TGFβ1 level at diagnosis, monitoring this level may be useful in detecting both disease persistence and recurrence after therapy. Ivanovic et al[25] examined the association between elevated plasma TGFβ1 level and disease progression in advanced breast cancer. Follow-up of six patients indicated a relationship between plasma TGFβ1 and treatment response.
In patients with cervical cancer treated with RT alone, pretreatment plasma TGFβ1 level is a significant prog-nostic factor for survival and local control, but not for radiation toxicity[26]. Using concurrent chemoradiotherapy, Yang et al[27] treated 42 patients with biopsy-proven squa-mous cell carcinoma or adenocarcinoma of the cervix, and assessed serum TGFβ1 level weekly. They have found that sudden elevation of serum TGFβ1 level after the first fraction of brachytherapy is accompanied by greater RT-related morbidity. Lower pretreatment TGFβ1 levels are associated with tumor response to chemoradiation. The conclusion is that serial changes in serum cytokines during chemoradiation may correlate with tumor regression and treatment morbidity.
In breast, gastric, colorectal, prostate, renal and liver cancers, a similar relationship to ours has been found between plasma TGFβ1 level and treatment response[28-31]. However, in advanced head and neck cancer and NSCLC, no similar relationship between plasma TGFβ1 level and tumor burden was found, and neither to treatment response[26]. Thus, for these cancers, it can be hypothesized that healthy tissues and the immune system are responsible for the major part of TGFβ1 production and that cancer cells make only minor contribution to the total plasma TGFβ1 level.
Among patients with local treatment failure and regional lymph node metastasis after RT, there were nine with increased serum TGFβ1 (Table 4). There was a positive relationship between changes in serum TGFβ1 level and local treatment failure and regional lymph node metastasis. All patients with increased serum TGFβ1 died within 18 months after RT, which strongly suggests that serum TGFβ1 level can predict progression in patients with esophageal carcinoma. Our results have been confirmed by other studies on different kinds of carcinoma[16,24].
To the best of our knowledge, few of these studies have shown the predictive power of TGFβ1 for disease progression in patients with esophageal carcinoma after RT. The present study demonstrates that a higher serum TGβ1 level after RT is strongly associated with residual or recurrent tumor or lymph node metastasis. These data suggest that serum TGFβ1 may be useful as a marker for monitoring tumor response to therapy and disease progression.
In conclusion, our findings provide preliminary evidence that significantly elevated serum TGFβ1 level in patients with esophageal carcinoma is associated with poor prognosis. Although the complete mechanism of action and the role of TGFβ1 in esophageal carcinoma remain to be elucidated, our results suggest that this biomarker may be useful for monitoring tumor response to therapy and diseases progression in patients with esophageal carcinoma.
The authors thank Professor Yi-Qin Du of the University of Pittsburgh Medical Center and Professor Ying-Song Xiang of the Department of Radiation Medicine of Shanghai Second Military Medical University for their assistance with data management and review of this manuscript. The authors also thank Gong Li MD of the Oncology Center, General Hospital of the People’s Armed Police Forces of Beijing, for review of this manuscript.
TGFβ1 is a cytokine with multiple biological functions. In recent studies, a relationship between prognosis of many tumors and this cytokine has also been found. These suggest that TGFβ1 may be a promising prognostic marker for some cancer patients. Based on these findings, we hypothesized that serial blood TGFβ1 measurements can be used to identify disease status in patients with esophageal carcinoma treated with conventional doses of radiotherapy (RT), and as a potential predictive marker that may allow us to stratify patients into different treatment groups.
A study has shown a significant correlation between TGFβ1 level measured in the azygos vein and distant lymph node metastasis in esophageal cancer. Few of studies have shown the predictive power of TGFβ1 for disease progression in patients with esophageal carcinoma after RT.
Our findings provide preliminary evidence that significantly elevated serum TGFβ1 level in patients with esophageal carcinoma is associated with poor prognosis.
TGFβ1 may be useful for monitoring tumor response to therapy and diseases progression in patients with esophageal carcinoma. It may allow us to stratify patients into different treatment groups.
Serum TGFβ1 level in esophageal carcinoma reflects disease status. A higher serum TGFβ1 level after RT is strongly associated with residual or recurrent tumor or lymph node metastasis.
This paper is a well done and interesting study regarding TGF-beta serum levels and response/survival to radiation therapy in patients with esophageal cancer. The science seems interesting and sound.
S- Editor Liu Y L- Editor Kerr C E- Editor Li HY
| 1. | Yamada S, Takai Y, Nemoto K, Ogawa Y, Kakuto Y, Hoshi A, Sakamoto K. Low-dose rate telecobalt therapy as a boost against esophageal carcinomas. Cancer. 1992;69:1099-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Smalley SR, Gunderson LL, Reddy EK, Williamson S. Radiotherapy alone in esophageal carcinoma: current management and future directions of adjuvant, curative and palliative approaches. Semin Oncol. 1994;21:467-473. [PubMed] |
| 3. | Wang Y, Shi XH, He SQ, Yao WQ, Wang Y, Guo XM, Wu GD, Zhu LX, Liu TF. Comparison between continuous accelerated hyperfractionated and late-course accelerated hyperfractionated radiotherapy for esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2002;54:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Gao XS, Qiao XY, Yang XR, Asaumi J, Zhou ZG, Wang YD, Zhou DA, Wan J, Kuroda M, Kishi K. Late course accelerated hyperfractionation radiotherapy concomitant with cisplatin in patients with esophageal carcinoma. Oncol Rep. 2002;9:767-772. [PubMed] |
| 5. | Zhao KL, Wang Y, Shi XH. Late course accelerated hyperfractionated radiotherapy for clinical T1-2 esophageal carcinoma. World J Gastroenterol. 2003;9:1374-1376. [PubMed] |
| 6. | Shi XH, Yao W, Liu T. Late course accelerated fractionation in radiotherapy of esophageal carcinoma. Radiother Oncol. 1999;51:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Zhao KL, Shi XH, Jiang GL, Yao WQ, Guo XM, Wu GD, Zhu LX. Late course accelerated hyperfractionated radiotherapy plus concurrent chemotherapy for squamous cell carcinoma of the esophagus: a phase III randomized study. Int J Radiat Oncol Biol Phys. 2005;62:1014-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Okunieff P, Cornelison T, Mester M, Liu W, Ding I, Chen Y, Zhang H, Williams JP, Finkelstein J. Mechanism and modification of gastrointestinal soft tissue response to radiation: role of growth factors. Int J Radiat Oncol Biol Phys. 2005;62:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Feltl D, Závadová E, Pála M, Hozák P. Post-treatment plasma transforming growth factor beta 1 (TGF-beta1) level predicts for late morbidity in patients with advanced head and neck cancer. Neoplasma. 2005;52:393-397. [PubMed] |
| 10. | Anscher MS, Kong FM, Marks LB, Bentel GC, Jirtle RL. Changes in plasma transforming growth factor beta during radiotherapy and the risk of symptomatic radiation-induced pneumonitis. Int J Radiat Oncol Biol Phys. 1997;37:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 103] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Evans ES, Kocak Z, Zhou SM, Kahn DA, Huang H, Hollis DR, Light KL, Anscher MS, Marks LB. Does transforming growth factor-beta1 predict for radiation-induced pneumonitis in patients treated for lung cancer? Cytokine. 2006;35:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Anscher MS, Marks LB, Shafman TD, Clough R, Huang H, Tisch A, Munley M, Herndon JE, Garst J, Crawford J. Risk of long-term complications after TFG-beta1-guided very-high-dose thoracic radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:988-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | De Jaeger K, Seppenwoolde Y, Kampinga HH, Boersma LJ, Belderbos JS, Lebesque JV. Significance of plasma transforming growth factor-beta levels in radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58:1378-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Pasche B. Role of transforming growth factor beta in cancer. J Cell Physiol. 2001;186:153-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Tsushima H, Ito N, Tamura S, Matsuda Y, Inada M, Yabuuchi I, Imai Y, Nagashima R, Misawa H, Takeda H. Circulating transforming growth factor beta 1 as a predictor of liver metastasis after resection in colorectal cancer. Clin Cancer Res. 2001;7:1258-1262. [PubMed] |
| 16. | Wu HS, Li YF, Chou CI, Yuan CC, Hung MW, Tsai LC. The concentration of serum transforming growth factor beta-1 (TGF-beta1) is decreased in cervical carcinoma patients. Cancer Invest. 2002;20:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Bierie B, Moses HL. TGF-beta and cancer. Cytokine Growth Factor Rev. 2006;17:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 325] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 18. | Fukai Y, Fukuchi M, Masuda N, Osawa H, Kato H, Nakajima T, Kuwano H. Reduced expression of transforming growth factor-beta receptors is an unfavorable prognostic factor in human esophageal squamous cell carcinoma. Int J Cancer. 2003;104:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Fukuchi M, Miyazaki T, Fukai Y, Nakajima M, Sohda M, Masuda N, Manda R, Tsukada K, Kato H, Kuwano H. Plasma level of transforming growth factor beta1 measured from the azygos vein predicts prognosis in patients with esophageal cancer. Clin Cancer Res. 2004;10:2738-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Anscher MS, Marks LB, Shafman TD, Clough R, Huang H, Tisch A, Munley M, Herndon JE, Garst J, Crawford J. Using plasma transforming growth factor beta-1 during radiotherapy to select patients for dose escalation. J Clin Oncol. 2001;19:3758-3765. [PubMed] |
| 21. | Leonard GD, McCaffrey JA, Maher M. Optimal therapy for oesophageal cancer. Cancer Treat Rev. 2003;29:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Song BC, Chung YH, Kim JA, Choi WB, Suh DD, Pyo SI, Shin JW, Lee HC, Lee YS, Suh DJ. Transforming growth factor-beta1 as a useful serologic marker of small hepatocellular carcinoma. Cancer. 2002;94:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Anscher MS, Kong FM, Andrews K, Clough R, Marks LB, Bentel G, Jirtle RL. Plasma transforming growth factor beta1 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 1998;41:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 195] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Kong FM, Washington MK, Jirtle RL, Anscher MS. Plasma transforming growth factor-beta 1 reflects disease status in patients with lung cancer after radiotherapy: a possible tumor marker. Lung Cancer. 1996;16:47-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Ivanović V, Todorović-Raković N, Demajo M, Nesković-Konstantinović Z, Subota V, Ivanisević-Milovanović O, Nikolić-Vukosavljević D. Elevated plasma levels of transforming growth factor-beta 1 (TGF-beta 1) in patients with advanced breast cancer: association with disease progression. Eur J Cancer. 2003;39:454-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Dickson J, Davidson SE, Hunter RD, West CM. Pretreatment plasma TGF beta 1 levels are prognostic for survival but not morbidity following radiation therapy of carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2000;48:991-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Yang YC, Wang KL, Su TH, Liao HF, Wu MH, Chen TC, Huang MC, Chen YJ. Concurrent cisplatin-based chemoradiation for cervical carcinoma: tumor response, toxicity, and serum cytokine profiles. Cancer Invest. 2006;24:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Maehara Y, Kakeji Y, Kabashima A, Emi Y, Watanabe A, Akazawa K, Baba H, Kohnoe S, Sugimachi K. Role of transforming growth factor-beta 1 in invasion and metastasis in gastric carcinoma. J Clin Oncol. 1999;17:607-614. [PubMed] |
| 29. | Kong FM, Anscher MS, Murase T, Abbott BD, Iglehart JD, Jirtle RL. Elevated plasma transforming growth factor-beta 1 levels in breast cancer patients decrease after surgical removal of the tumor. Ann Surg. 1995;222:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 170] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Shariat SF, Shalev M, Menesses-Diaz A, Kim IY, Kattan MW, Wheeler TM, Slawin KM. Preoperative plasma levels of transforming growth factor beta(1) (TGF-beta(1)) strongly predict progression in patients undergoing radical prostatectomy. J Clin Oncol. 2001;19:2856-2864. [PubMed] |
| 31. | Robson H, Anderson E, James RD, Schofield PF. Transforming growth factor beta 1 expression in human colorectal tumours: an independent prognostic marker in a subgroup of poor prognosis patients. Br J Cancer. 1996;74:753-758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 3.0] [Reference Citation Analysis (0)] |