Published online Oct 21, 2007. doi: 10.3748/wjg.v13.i39.5232
Revised: August 4, 2007
Accepted: September 1, 2007
Published online: October 21, 2007
AIM: To observe whether pancreatic and duodenal homeobox factor-1 enhances the differentiation of pancreatic ductal epithelial cells into insulin-producing cells in vitro.
METHODS: Rat pancreatic tissue was submitted to digestion by collagenase, ductal epithelial cells were separated by density gradient centrifugation and then cultured in RPMI1640 medium with 10% fetal bovine serum. After 3-5 passages, the cells were incubated in a six-well plate for 24 h before transfection of recombination plasmid XlHbox8VP16. Lightcycler quantitative real-time RT-PCR was used to detect the expression of PDX-1 and insulin mRNA in pancreatic epithelial cells. The expression of PDX-1 and insulin protein was analyzed by Western blotting. Insulin secretion was detected by radioimmunoassay. Insulin-producing cells were detected by dithizone-staining.
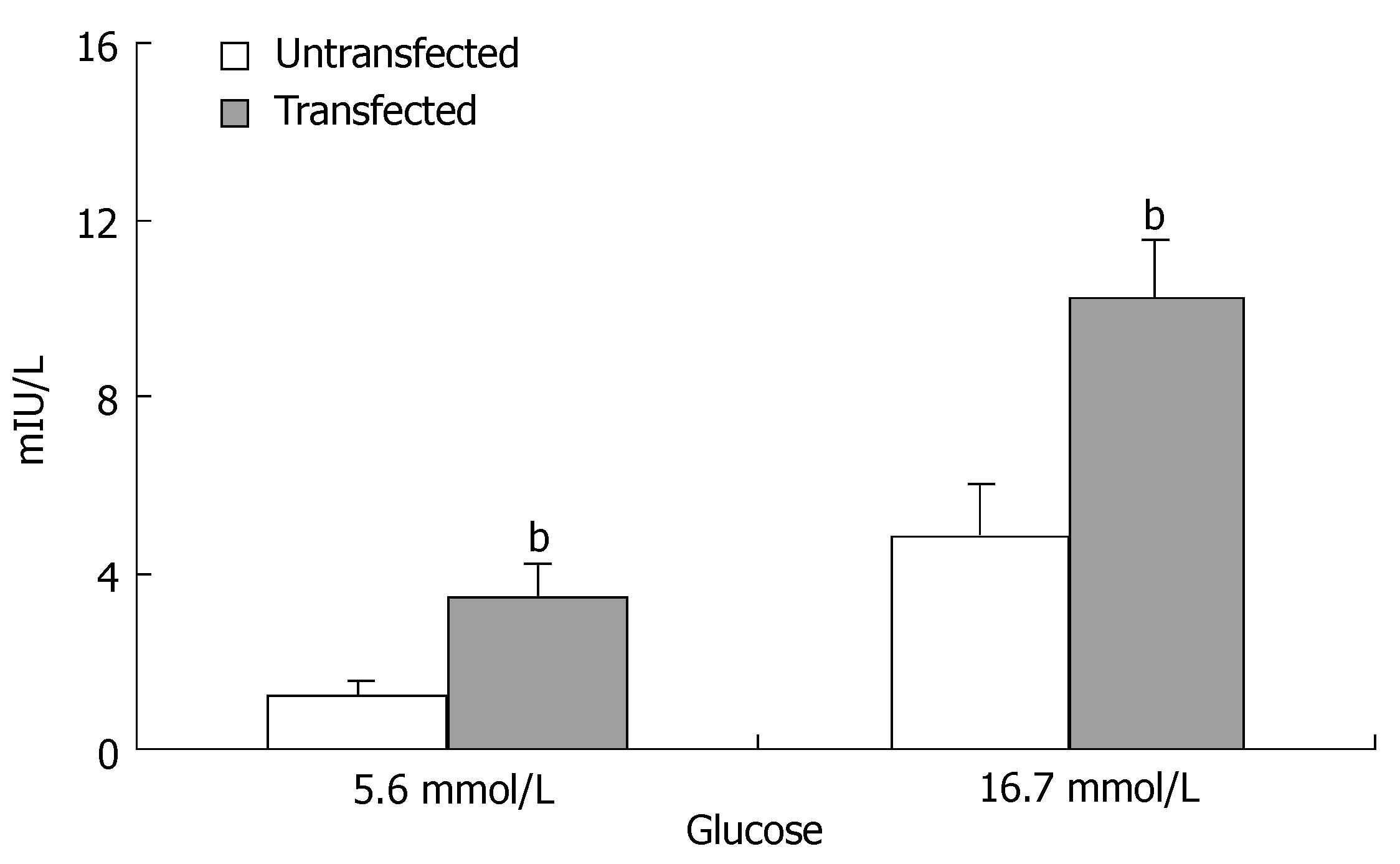
RESULTS: XlHbox8 mRNA was expressed in pancreatic ductal epithelial cells. PDX-1 and insulin mRNA as well as PDX-1 and insulin protein were significantly increased in the transfected group. The production and insulin secretion of insulin-producing cells differentiated from pancreatic ductal epithelial cells were higher than those of the untransfected cells in vitro with a significant difference (1.32 ± 0.43 vs 3.48 ± 0.81, P < 0.01 at 5.6 mmol/L; 4.86 ± 1.15 vs 10.25 ± 1.32, P < 0.01 at 16.7 mmol/L).
CONCLUSION: PDX-1 can differentiate rat pancreatic ductal epithelial cells into insulin-producing cells in vitro. In vitro PDX-1 transfection is a valuable strategy for increasing the source of insulin-producing cells.
-
Citation: Liu T, Wang CY, Yu F, Gou SM, Wu HS, Xiong JX, Zhou F.
In vitro pancreas duodenal homeobox-1 enhances the differentiation of pancreatic ductal epithelial cells into insulin-producing cells. World J Gastroenterol 2007; 13(39): 5232-5237 - URL: https://www.wjgnet.com/1007-9327/full/v13/i39/5232.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i39.5232
Diabetes mellitus is a common endocrine and metabolic disease in the world. At present, insulin injection therapy is used in the treatment of type 1 and some type 2 diabetes. However, the results are not satisfactory. Transplantation of islets is one of the promising therapies for type 1 diabetes and can effectively prevent diabetic nephropathy, retinopathy and other complications[1]. Since it is limited by the shortage of islets, expanding the sources of islet cells (especially beta cells) has become a hot field of research in pancreas transplantation.
In recent years, great efforts have been made to differentiate embryonic stem cells, pancreatic ductal epithelial multipotent progenitor cells and bone marrow stem cells into islet cells[2-4]. However, it was reported that the most promising way is to differentiate pancreatic stem cells into islet cells, because the process of cell differentiation and growth is shorter than that of bone marrow stem cells and embryonic stem cells, and the induction method in vitro is relatively simple[5]. The amount of islets differentiated from pancreatic stem cells in vitro is minimal and insulin released by islets is insufficient to meet the clinical needs[6]. How to enhance the efficiency of differentiation in vitro and increase the output of insulin-producing cells and insulin-release needs to be studied.
The pancreatic and duodenal homeobox factor-1 (PDX-1), also known as islet/duodenum homeobox-1/somatostatin-DE transactivating factor 1/insulin promoter factor DE 1, a homeodomain containing transcription factor, is homologous to a Xenopus endoderm-specific homeodomain protein, XlHbox 8. It plays a central role in regulating pancreatic development and insulin gene transcription[7]. Transfection of PDX-1 gene into rat intestinal epithelial cell line IEC-6 can produce insulin[8]. Ferberr et al[9] showed that PDX-1 can endow some cells in the liver with pancreatic beta-cell characteristics in vivo using recombinant adenovirus-mediated gene delivery. Bonner-Weir et al[10] reported that PDX-1 protein can permeate pancreatic duct and islet cells due to an Antennapedia-like protein transduction domain sequence in its structure and transduced PDX-1 functions similarly to endogenous PDX-1. PDX-1 protein transduction is a safe and valuable strategy for enhancing insulin gene transcription and facilitating differentiation of ductal progenitor cells into insulin-producing cells without gene transfer technology. Recently, Yamada et al[11] showed that mature liver cells can also be induced into insulin-producing cells by in vitro PDX-1 gene transfection. These studies have highlighted the potential usefulness of PDX-1 as a reprogramming factor of non-beta-cells toward beta-cell-like cells that can be used in diabetes cell/gene therapy.
Due to the high homology of PDX-1 sequence in different species[12], we investigated the role of exogenous PDX-1 in pancreatic ductal epithelial cells in adult rats by transfecting exogenous PDX-1 (XlHbox8) into pancreatic ductal epithelial cells in vitro. The results demonstrate that we can enhance the differentiation of pancreatic ductal epithelial cells into insulin-producing cells and insulin-release via PDX-1 (XlHbox8-VP16) transfection.
Rat (adult male S-D rats, weighing 250-300 g) pancreatic tissue was digested in 1 g/L type V collagenase (Sigma) and incubated at 37°C for 40 min with intermittent shaking and then terminated by Hank’s solution, followed by centrifugation at 1000 r/min for 5 min. After purification on a Ficoll gradient, 50%-95% islets were found on the top interface (1.062/1.096 densities) with varying amounts of duct and degranulated acinar tissues, and 1%-15% islets were found on the middle interface (1.096/1.11 densities). Duct, degranulated acini and pellet were composed of well-granulated acinar tissue with less than 1% islets. In the top and middle layers, there were sheets of ductal epithelium from larger ducts whereas the clumps of exocrine cells found in all layers consisted of small intercalated ducts continuing into the acini. The epithelial cells were washed 3 times with Hank’s solution containing 5% fetal bovine serum (FBS, Gibco), then put into RMPI 1640 culture medium (Hyclone) containing 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cell suspensions were put into non-treated T-75 flasks and incubated at 37°C in an atmosphere containing 5% CO2. After 48 h, the nonadherent tissue (both viable and dead) was removed. The medium was changed. The adherent or residual cells were expanded for up to 1 wk with media changed every 2-3 d. The adherent or residual cells were continuously cultured in an atmosphere containing 5% CO2 and 95% humidity. After 3-5 passages, the ductal epithelial cells were used in experiments.
Plasmid pCS2-TTR-XlHbox8VP16 was kindly provided by Professor J M.W.Slack (Centre for Regenerative Medicine, Department of Biology and Biochemistry, University of Bath, Bath)[13]. Plasmids were transfected into pancreatic ductal epithelial cells with Lipofectamine 2000 (Invitrogen) transfection reagent according to the manufacturer's instructions. The medium was replaced with a fresh medium after 24 h of transfection. The cells were cultured for a further 7 d, and analyzed by reverse transcriptase polymerase chain reaction (RT-PCR) or Western blotting at variable time points.
To induce differentiation of pancreatic ductal epithelial cells to insulin-producing cells, forty-eight hours after transfection, pancreatic ductal epithelial cells were transferred to a medium supplemented with 200 μg/mL G418, 10 mmol/L nicotinamide, and insulin/transferrin/selenium(ITS, Sigma). G418-resistant colonies were found about 4 wk after transfection. The resulting clusters were cultured for 1-5 d in RPMI 1640 supplemented with 10% FBS, 10 mmol/L nicotinamide, 200 μg/mL G418, 100 IU/mL penicillin, 0.1 mg/mL streptomycin, and 5.6 mmol/L glucose[2].
Fifty mg pancreatic tissues and 1 mL TRIzol were mixed with 0.3 mL chloroform added, and then centrifuged. Isopropanol (0.5 mL) was added to supernatant and centrifuged with supernatant discarded. Deposit was washed with 70% ethanol, and dissolved in DEPC-treated water to obtain total mRNA. In RT-PCR, 4 μL of mRNA and 0.5 μL of Oligo (dt) were added to 6.5 μL of distilled water. After annealing at 70°C for 5 min and immediate cooling on ice, 4.0 μmol/L of 5 × first strand buffer, 2.0 μL of 10 mmol/L dNTP, 0.5 μL of RNasin and 0.5 μL RTase were added to get a total reaction volume of 20 μL. The reaction was allowed to proceed at 37°C for 60 min, followed by at 95°C for 5 min to inactivate the enzyme. RT-PCR assay was performed three times for each cDNA sample. The total PCR volume consisted of 1 μL of cDNA, 1 μL of SYBRGreen PCR I, 5 μL 10 × buffer, 1.6 μL of primers (rat PDX-1: sense 5′-CTTGGGTATGGATCTGTGG-3′ and antisense 5′-CGGACTCATCGTACTCCTGCTT-3′; Xenopus PDX-1 homologue XlHbox8: sense 5′-TGCCAACTTCATCCCAGCCC-3′ and antisense 5′-GGCAGATGAAGAGGGCTC-3′; insulin: sense 5′-GCTACAATCATAGACCATC-3′ and antisense 5′-GGCGGGGAGTGGTGGACTC-3′; beta-actin: sense 5′-CTTGGGTATGGAATCCTGTGG-3′ and antisense 5′-CGGACTCATCGTACTCCTGCTT-3′), 7 μL of MgCl2, 0.5 μL of Taq DNA polymerase, 1 μL of dNTP and 33 μL of distilled water. After denaturation of the enzyme at 94°C for 2 min, 45 cycles of PCR assay were carried out, with denaturation at 94°C for 30 s, annealing at 57°C for 30 s and extension at 72°C for 30 s. Fluorometric PCR was performed with the FTC-2000 system. The expression of each transporter protein gene determined was relative to the β-actin RNA gene.
Total protein was obtained as previously described[14] at various time points (d 0, 1, 3, 5, 7, 14, 21, 28 after transfection). Proteins in samples were separated by polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane and blocked overnight in a blocking solution (5% dry milk in PBS). The membrane was incubated at 37°C for 1 h with goat anti PDX-1 antibody (1:500, Santa Cruz) and goat anti insulin antibody (1:500, Santa Cruz), washed with PBS and incubated at 37°C for 1 h with horseradish peroxidase-labeled secondary antibody (1:2500). Finally, proteins were visualized on a film with the ECL method.
A dithizone (DTZ; Sigma) stock solution was prepared with 50 mg of DTZ in 5 mL of dimethylsulfoxide (DMSO) and stored at -15°C[15]. In vitro DTZ staining was performed by adding 10 μL of the stock solution to 1 mL of culture medium. The staining solution was filtered through a 0.2 μm filter and used as the DTZ working solution which was added to culture dishes and incubated at 37°C for 15 min. Clusters were examined under a phase contrast microscope.
Differentiation of cells transfected with or without TTR-XlHbox8VP16 was induced with either normal dose glucose (5.5 mmol/L) or high dose glucose (16.7 mmol/L). Insulin concentration was measured using an insulin RIA kit (Linco).
The data were expressed as mean ± SD. Individual treatment was compared using Student’s t-test and ANOVA. P < 0.05 was considered statistically significant.
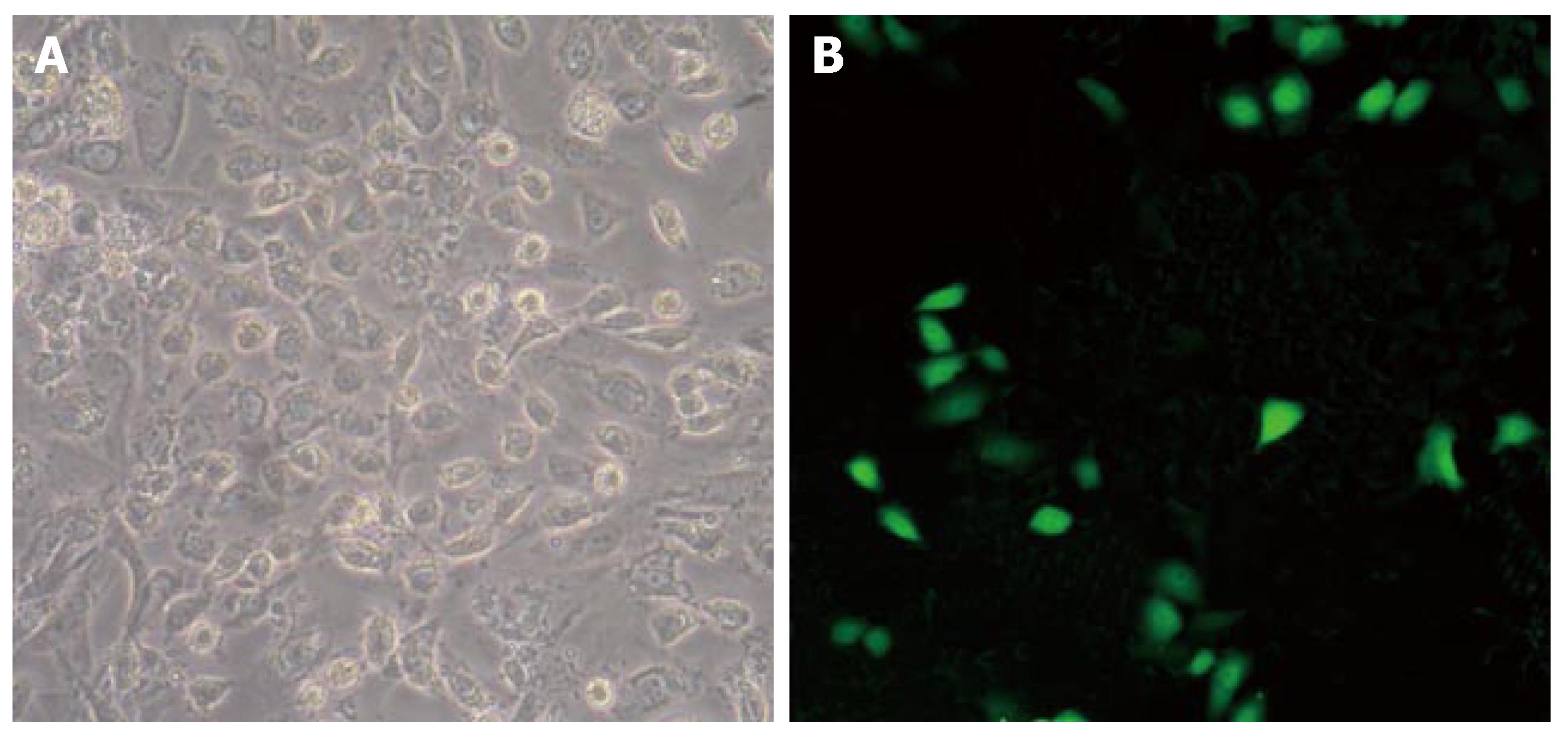
Green fluorescence could be seen in pancreatic ductal epithelial cells transfected with XlHbox8. The distribution of green fluorescence in nuclei and cytoplasm indicated the characteristics of location of cytokines in these cells. Twenty-four hours after transfection, the expression of GFP fluorescence was observed under fluorescence microscope (Figure 1). Green fluorescent cells were calculated. The transfection efficiency of pancreatic ductal epithelial cells was 3%-5%. These results suggest that exogenous XlHbox8 could be transfected into nuclei and cytoplasm of pancreatic ductal epithelial cells.
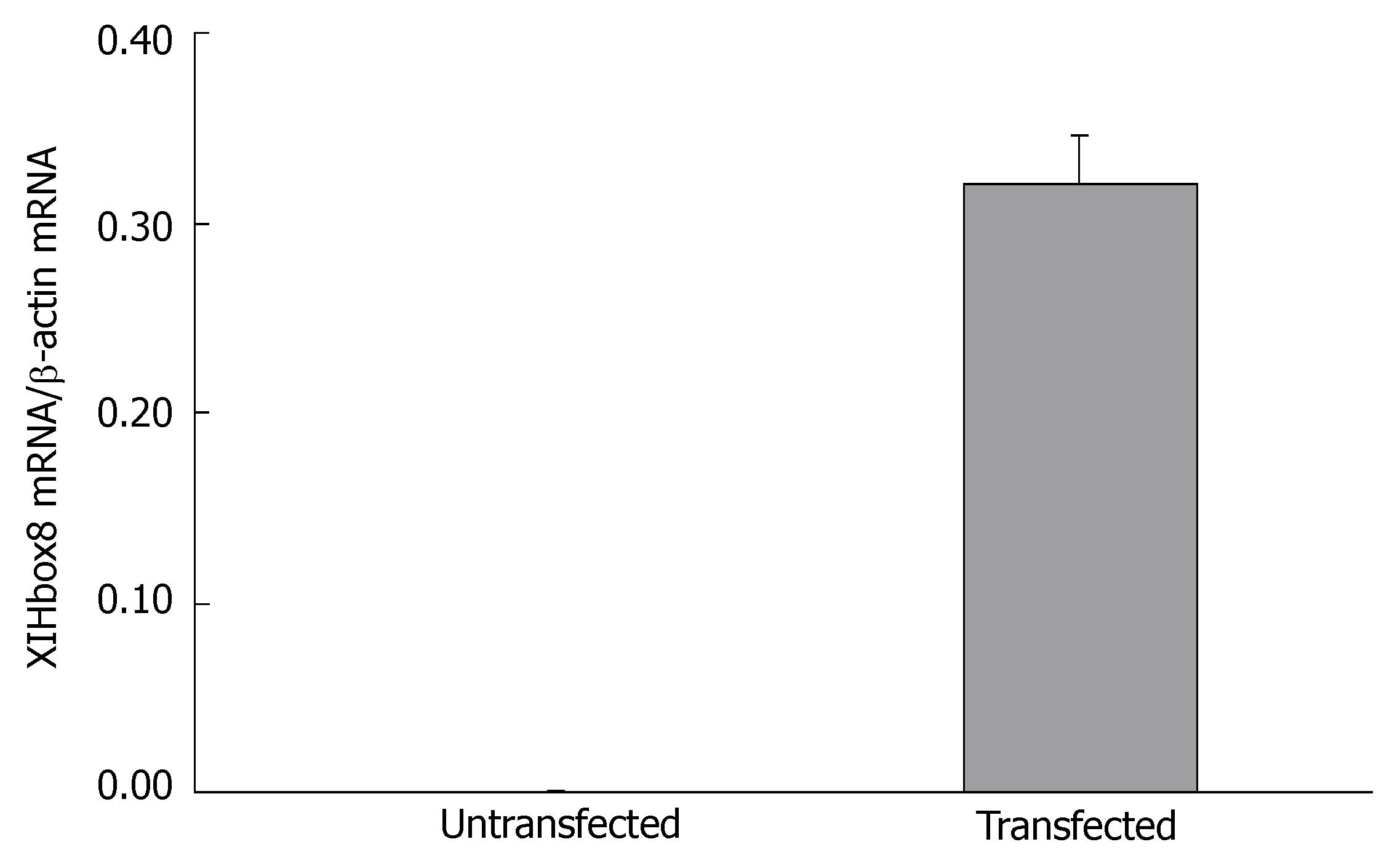
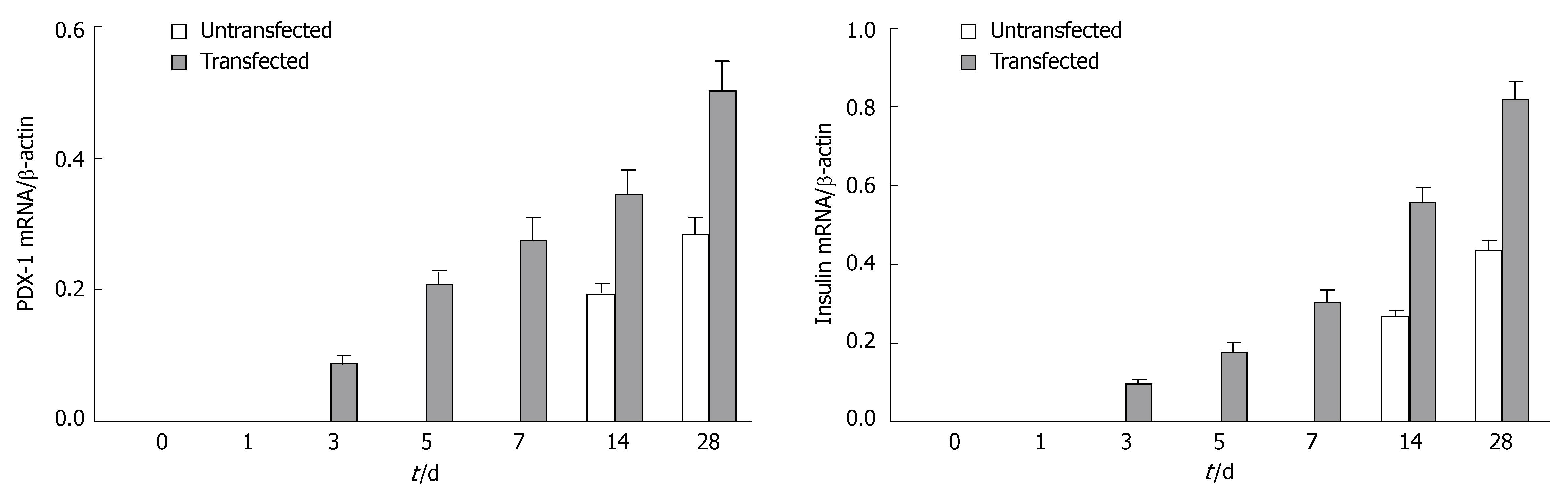
To investigate the change in gene expression patterns caused by exogenous PDX-1 (XlHbox8) expression in pancreatic ductal epithelial cells, we performed RT-PCR analysis. As shown in Figure 2, the cells transfected with TTR-XlHbox8VP16 showed the expression of XlHbox8 mRNA, which was not detectable in the untransfected cells. In addition, the results also showed that the expression of PDX-1 and insulin mRNA increased between the 1st and 7th d after transfection (Figure 3), which is consistent with the reported results[13]. In contrast, the expression of PDX-1 and insulin mRNA was not detectable in the untransfected cells.
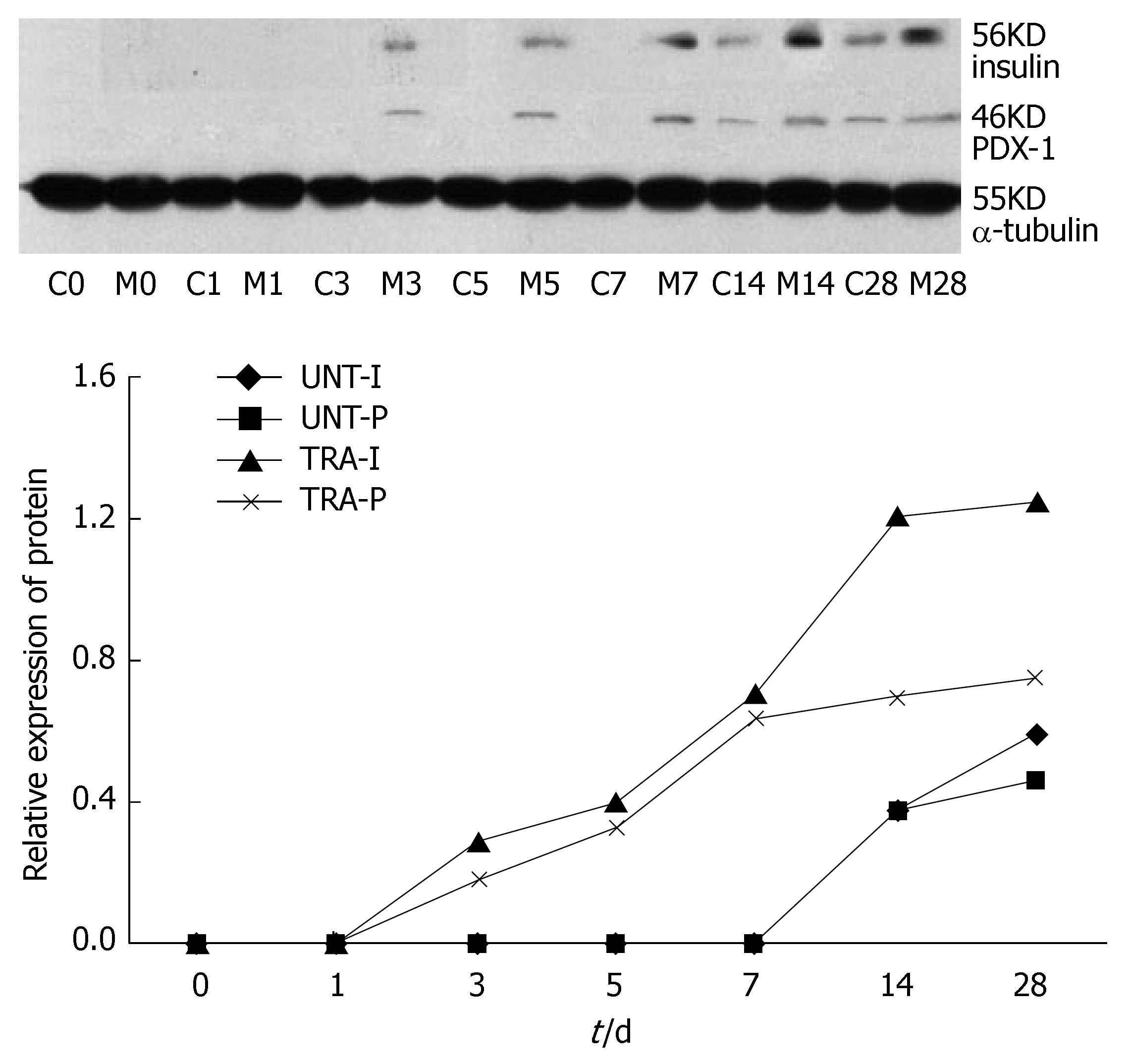
Western blotting showed that PDX-1 and insulin protein were expressed in the transfected cells but not in the untransfected cells. After induction, the untransfected cells also began to express PDX-1 and insulin protein. Moreover, the expression increased between the 14th and 28th d in the transfected cells. The expression of PDX-1 and insulin protein in the transfected cells increased more obviously (Figure 4).
These results suggest that once XlHbox8 was transfected into pancreatic ductal epithelial cells, endogenous PDX-1 gene transcription was amplified by this XlHbox8 and might enhance differentiation of pancreatic ductal epithelial cells into insulin-producing cells.
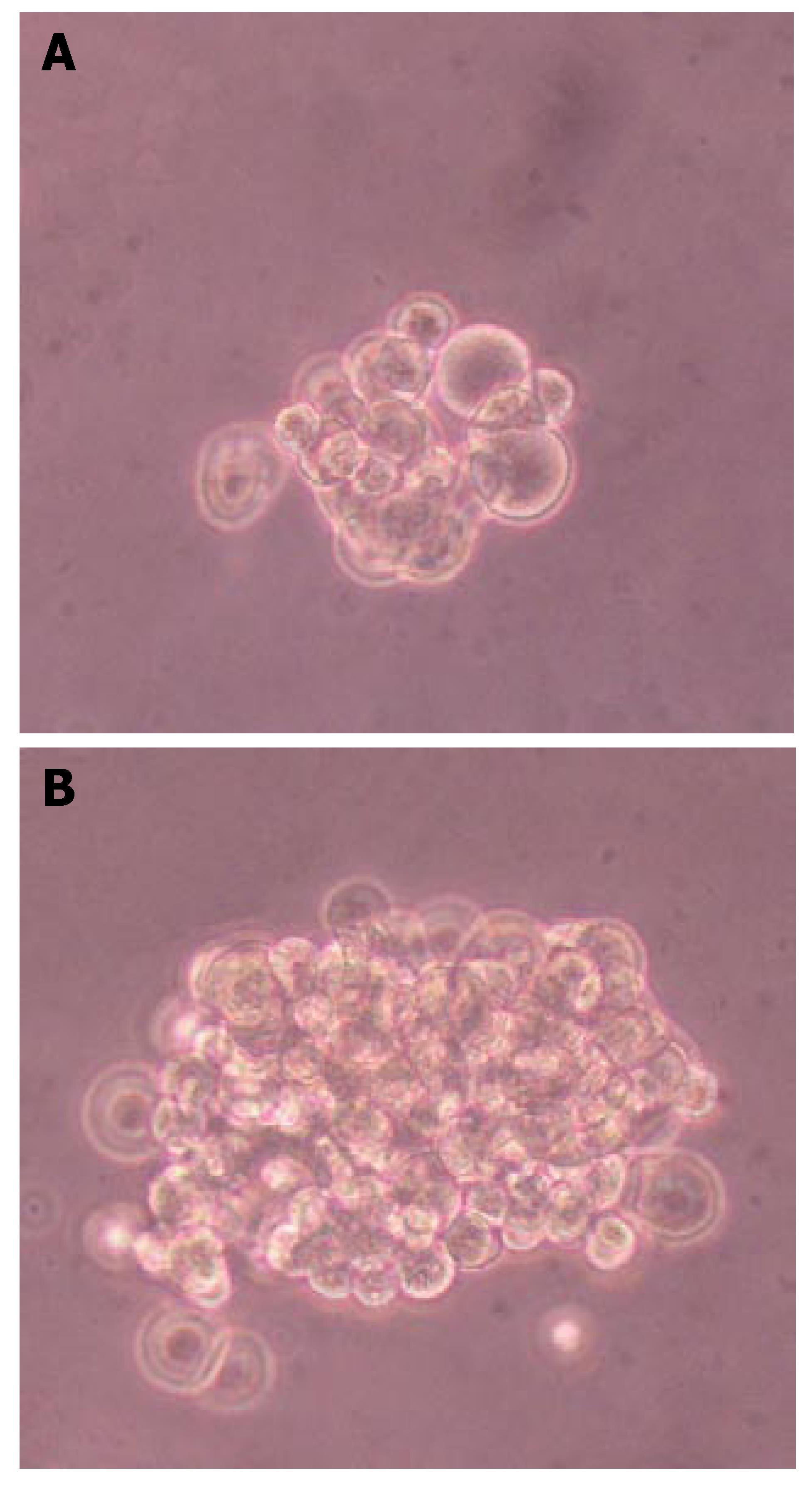
Pancreatic ductal epithelial cells were transfected with XlHbox8 expression plasmid, and stable transfectants were selected according to G418 resistance. Further differentiation of pancreatic ductal epithelial cells was induced in serum-free medium supplemented with ITS, keratinocyte growth factor (KGF, Sigma) and basic fibroblast growth factor (bFGF, Sigma). During induction, most insulin-producing cells could be seen under microscope. Dithizone staining showed more insulin-producing cells in the transfected group than in the untransfected group (Figure 5).
The most important and characteristic property of insulin-producing cells is their ability to secrete insulin in response to an elevated external glucose concentration. We examined the insulin concentration in the medium of differentiated cells after challenged with 5.6 and 16.7 mmol/L glucose, using a RIA kit (Figure 6). The results showed that insulin secretion was significantly increased in the transfected group compared with the untransfected group.
Pancreas duodenum homeobox-1 (PDX-1) is also known as islet/duodenal homeobox-1(IDX-1), insulin promoter factor-1 (IPF-1), insulin upstream factor-1 (IUF-1), somatostatin transactivating factor-1 (STF-1) and glucose-sensitive factor (GSF)[7]. PDX-1 is a transcription factor encoded by Hox-like homeodomain gene and also one of the specific signals of pancreatic stem cells. Human PDX-1 gene consists of two exons and spans a region of about 6 kb in humans and 282 amino acids[16]. In mice lacking PDX-1, pancreas development is blocked at its very early stage, and there are no or diminished endocrine cells in the rostral duodenum and stomach[17,18], suggesting that PDX-1 plays a very important role in the development and maintenance of normal pancreatic beta cell function, which also has a potential value in gene therapy for diabetes. Amplifying and inducing differentiation of non-islet cells into insulin-producing cells by PDX-1 has become a hot field of study. Miyazaki et al[19] reported that islet-like cellular clusters are significantly increased and insulin-producing cells can release more insulin by transfecting exogenous PDX-1 into embryonic stem cells. Further investigation found that PDX-1 can enhance the expression of insulin 2, somatostatin and HNF6 genes in the differentiated cells[19]. XlHbox8 is the homologous of PDX-1. Horb et al[20] transfected Xlhbox8-VP16 into liver cells and found that some liver cells could be trans-differentiated into pancreatic cells.
Bonner-Weir et al[3] reported human pancreatic ductal epithelial cells can expand and differentiate into islet cells in vitro. In this study, most of early adherent cells and a few fibroblasts were found to be pancreatic ductal epithelial cells. PDX-1 may express in the whole pancreas of an early embryo. However, in pancreas of adult rats, PDX-1 expresses primarily in beta and delta cells, but not in pancreatic ductal epithelium. PDX-1 can also express in activated pancreatic ductal epithelial cells[12,14]. When pancreatic ductal epithelial cells are transfected with exogenous homologous of PDX-1 and XlHbox8, endogenous PDX-1 gene can be activated, increasing insulin mRNA and protein expression[9], suggesting that once XlHbox8 is transfected into pancreatic ductal epithelial cells, endogenous PDX-1 gene transcription is activated by XlHbox8 and insulin gene, thus enhancing its expression. In our study, the expression of insulin mRNA and protein was enhanced after treatment with exogenous XlHbox8, indicating that XlHbox8 may promote differentiation of pancreatic ductal epithelial cells into insulin-producing cells. When pancreatic ductal epithelial cells were transfected with exogenous PDX-1, endogenous PDX-1 gene transcription was amplified, promoting differentiation of pancreatic ductal epithelial cells into insulin-producing cells, which is in agreement with the reported results[10]. In the present study, after XlHbox-VP16 was transfected into pancreatic ductal epithelial cells, differentiation of pancreatic ductal epithelial cells to insulin-producing cells was significantly increased, suggesting that this method can produce more insulin-producing cells. It was reported that unmodified PDX-1 is insufficient to activate transcription in the absence of other cofactors, such as Pbx and Meis[20]. Beta cells are able to secrete insulin in response to an elevated external glucose concentration. In this study, the results of RIA show that the treated cells can produce more insulin than the untreated cells. However, whether differentiated insulin-producing cells have their physiological function in vivo still needs to be further tested in vivo. Moreover, the low transfection efficiency also limits the production of insulin-producing cells.
In conclusion, PDX-1 (XIHbox8) can significantly promote in vitro differentiation of pancreatic ductal epithelial cells into insulin-producing cells. This new method can be used in the treatment of diabetes.
The authors are grateful to the technical assistance from Yuan Tian and Jin-Hui Zhang at Research Laboratory of General Surgery, Union Hospital, Wuhan.
Diabetes mellitus is a common endocrine and metabolic disease in the world. Although transplantation of islets is one of the promising therapies for type 1 diabetes, its application is limited due to the shortage of islets.
Expanding the sources of islet cells (especially beta cell) is a hot field of research in pancreas transplantation. Great efforts have made to differentiate embryonic stem cells, pancreatic ductal epithelial progenitor cells and bone marrow stem cells into islet cells. The most promising method is to differentiate pancreatic stem cells into islet cells, but the amount of islets obtained through differentiation of pancreatic ductal cells in vitro is minimal and insulin released by islets is insufficient.
The pancreatic and duodenal homeobox factor-1 (PDX-1) plays a central role in regulating pancreatic development and insulin gene transcription. In this study, we transfected PDX-1 (XlHbox8VP16) plasmid into human pancreatic ductal cells.
PDX-1 (XIHbox8) can significantly promote in vitro differentiation of pancreatic ductal epithelial cells into insulin-producing cells. This new method may be used in the treatment of diabetes.
This manuscript provides direct evidence for the enhanced differentiation of pancreatic ductal cells into insulin-producing cells. The study is well designed and the results are convincing.
S- Editor Zhu LH L- Editor Wang XL E- Editor Lu W
| 1. | Bertuzzi F, Secchi A, Di Carlo V. Islet transplantation in type 1 diabetic patients. Transplant Proc. 2004;36:603-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Soria B, Roche E, Berná G, León-Quinto T, Reig JA, Martín F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes. 2000;49:157-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 635] [Cited by in F6Publishing: 570] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 3. | Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O'Neil JJ. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA. 2000;97:7999-8004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 751] [Cited by in F6Publishing: 808] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 4. | Chen LB, Jiang XB, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol. 2004;10:3016-3020. [PubMed] [Cited in This Article: ] |
| 5. | Serup P, Madsen OD, Mandrup-Poulsen T. Islet and stem cell transplantation for treating diabetes. BMJ. 2001;322:29-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Hussain MA, Theise ND. Stem-cell therapy for diabetes mellitus. Lancet. 2004;364:203-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Hui H, Perfetti R. Pancreas duodenum homeobox-1 regulates pancreas development during embryogenesis and islet cell function in adulthood. Eur J Endocrinol. 2002;146:129-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Yoshida S, Kajimoto Y, Yasuda T, Watada H, Fujitani Y, Kosaka H, Gotow T, Miyatsuka T, Umayahara Y, Yamasaki Y. PDX-1 induces differentiation of intestinal epithelioid IEC-6 into insulin-producing cells. Diabetes. 2002;51:2505-2513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 573] [Cited by in F6Publishing: 535] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 10. | Noguchi H, Kaneto H, Weir GC, Bonner-Weir S. PDX-1 protein containing its own antennapedia-like protein transduction domain can transduce pancreatic duct and islet cells. Diabetes. 2003;52:1732-1737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Yamada S, Yamamoto Y, Nagasawa M, Hara A, Kodera T, Kojima I. In vitro transdifferentiation of mature hepatocytes into insulin-producing cells. Endocr J. 2006;53:789-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Ashizawa S, Brunicardi FC, Wang XP. PDX-1 and the pancreas. Pancreas. 2004;28:109-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Li WC, Horb ME, Tosh D, Slack JM. In vitro transdifferentiation of hepatoma cells into functional pancreatic cells. Mech Dev. 2005;122:835-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Sharma A, Zangen DH, Reitz P, Taneja M, Lissauer ME, Miller CP, Weir GC, Habener JF, Bonner-Weir S. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999;48:507-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 262] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Shiroi A, Yoshikawa M, Yokota H, Fukui H, Ishizaka S, Tatsumi K, Takahashi Y. Identification of insulin-producing cells derived from embryonic stem cells by zinc-chelating dithizone. Stem Cells. 2002;20:284-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Yokoi N, Serikawa T, Walther R. Pdx1, a homeodomain transcription factor required for pancreas development, maps to rat chromosome 12. Exp Anim. 1997;46:323-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1357] [Cited by in F6Publishing: 1303] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 18. | Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983-995. [PubMed] [Cited in This Article: ] |
| 19. | Miyazaki S, Yamato E, Miyazaki J. Regulated expression of pdx-1 promotes in vitro differentiation of insulin-producing cells from embryonic stem cells. Diabetes. 2004;53:1030-1037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 201] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Horb ME, Shen CN, Tosh D, Slack JM. Experimental conversion of liver to pancreas. Curr Biol. 2003;13:105-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 222] [Article Influence: 10.6] [Reference Citation Analysis (0)] |