Published online Oct 21, 2007. doi: 10.3748/wjg.v13.i39.5208
Revised: July 26, 2007
Accepted: August 10, 2007
Published online: October 21, 2007
AIM: To investigate the role of small intestinal carcinoid tumor-derived fibrotic mediators, TGFβ1 and CTGF, in the mediation of fibrosis via activation of an “intestinal” stellate cell.
METHODS: GI carcinoid tumors were collected for Q RT-PCR analysis of CTGF and TGFβ1. Markers of stellate cell desmoplasia were identified in peritoneal fibrosis by immunohistochemistry and stellate cells cultured from fresh resected fibrotic tissue. CTGF and TGFβ1 were evaluated using quantitative tissue array profiling (AQUA analysis) in a GI carcinoid tissue microarray (TMA) with immunostaining and correlated with clinical and histologically documented fibrosis. Serum CTGF was analyzed using a sandwich ELISA assay.
RESULTS: Message levels of both CTGF and TGFβ1 in SI carcinoid tumors were significantly increased (> 2-fold, P < 0.05) versus normal mucosa and gastric (non-fibrotic) carcinoids. Activated stellate cells and markers of stellate cell-mediated fibrosis (vimentin, desmin) were identified in histological fibrosis. An intestinal stellate cell was immunocytochemically and biochemically characterized and its TGFβ1 (10-7M) initiated CTGF transcription response (> 3-fold, P < 0.05) demonstrated. In SI carcinoid tumor patients with documented fibrosis, TMA analysis demonstrated higher CTGF immunostaining (AQUA Score: 92 ± 8; P <0.05), as well as elevated TGFβ1 (90.6 ± 4.4, P < 0.05). Plasma CTGF (normal 12.5 ± 2.6 ng/mL) was increased in SI carcinoid tumor patients (31 ± 10 ng/mL, P < 0.05) compared to non-fibrotic GI carcinoids (< 15 ng/mL).
CONCLUSION: SI carcinoid tumor fibrosis is a CTGF/TGFβ1-mediated stellate cell-driven fibrotic response. The delineation of the biology of fibrosis will facilitate diagnosis and enable development of agents to obviate its local and systemic complications.
- Citation: Kidd M, Modlin I, Shapiro M, Camp R, Mane S, Usinger W, Murren J. CTGF, intestinal stellate cells and carcinoid fibrogenesis. World J Gastroenterol 2007; 13(39): 5208-5216
- URL: https://www.wjgnet.com/1007-9327/full/v13/i39/5208.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i39.5208
Carcinoid (neuroendocrine) tumors are enigmatic, generally slow growing malignancies that occur most frequently (67%) in the GI tract[1]. They are not rare lesions, arising in 1.68 of every 100000 people[1]. The commonest gut tumor is the SI carcinoid tumor[1,2], which is derived from neuroendocrine enterochromaffin (EC) cells. SI carcinoid tumors are usually identified based on their characteristic paroxysmal symptomatology of flushing, sweating and diarrhea. They are often, however, detected at surgery for unexplained bowel obstruction[3], as a consequence of the fibrosis that they engender[4]. The etiology of this desmoplastic response is unknown but is a consequence of conversion of the normally filmy and flexible mesentery into a contracted fibrous adhesive mass with bands and even retroperitoneal desmoplasia[5,6]. These events are due both to tumor invasion and the ability of secretory products of the EC cell to initiate fibrosis by activating local cells to produce a desmoplastic response[7]. SI carcinoid tumor patients also develop distant (cardiac) fibrosis suggesting that the bioactive agents involved in the process have both a paracrine and a systemic effect[6]. In contrast, neither gastric carcinoids (derived from the neuroendocrine EC-like (ECL) cell) nor pulmonary carcinoids are associated with extensive local or systemic desmoplastic responses[8].
The mechanism whereby such fibrosis occurs is unknown although serotonin has previously been suggested as a mediator[6]. TGFβ1 and CTGF are well-characterized fibrotic factors[9-12]. TGFβ1 is a profibrotic mediator that induces CTGF expression[10]. Together, these factors stimulate over-production of collagen synthesis[13,14]. The target cells of TGFβ1 and CTGF are activated myofibroblasts, also known as stellate cells[15,16]. In the pancreas, TGFβ1 activates pancreatic stellate cells (PSCs) in both experimental and human pancreatic fibrosis; these cells are the main cellular source of collagen in chronic pancreatitis[17-19]. SI neuroendocrine tumors express TGFβ1 and its receptors, while stromal cellular elements around tumor nests express the TGFβ receptor[20]. This suggests a mechanism by which tumor cells can interact with and alter the character of the surrounding stroma.
We hypothesized that tumor TGFβ1 and CTGF produced by EC cells is involved in the mechanism of SI carcinoid tumor fibrosis via activation of an “intestinal” stellate cell. The aims of this study were to: (1) quantify CTGF and TGFβ1 message in carcinoid tumor tissue; (2) examine protein expression levels of CTGF and TGFβ1 and matrix proteins using immunohistochemistry in SI carcinoid tumors and intestinal fibrosis; (3) isolate and characterize the “intestinal” stellate cell; (4) examine the effects of TGFβ1 on this cell type; (5) quantitatively analyze CTGF and TGFβ1 protein levels on a GI carcinoid tissue microarray by AQUA analysis; and 6) determine whether serum CTGF discriminated SI carcinoid tumor patients with fibrosis from other non-fibrotic GI carcinoids.
These studies were approved by the Human Investigations Committee at the Yale University School of Medicine.
Tissue for molecular analysis: Tumor tissue from ten GI carcinoid patients (M:F = 6:4; median age [range] = 60 years [40-78]) diagnosed with either SI EC cell carcinoid tumors (n = 5) or gastric ECL cell carcinoids (n = 5) were collected for this study (Table 1). None of the patients had received therapy (surgery or somatostatin analogues) prior to tissue procurement. Paired normal tissue samples were also obtained from adjacent, macroscopically normal, non-tumor mucosa in nine cases from these patients.
Tissue for cell culture analysis: Tumor tissue and mesenteric fibrotic tissue was obtained from a patient with a fibrotic SI carcinoid tumor (male, 43 years; sample #6) operated on at Yale University (by IMM). This patient had not received medical therapy (somatostatin analogues) prior to surgery and was a de novo case of SI fibrosis.
GI Carcinoid TMA: Formalin-fixed paraffin-embedded tissue blocks containing GI carcinoids (stomach: n = 7; and SI: n = 36) diagnosed between 1965 and 2001 at the Yale University School of Medicine Department of Pathology were retrieved. Follow-up information was available (median follow-up: 110 mo, range: 24-456 mo) for all patients. The TMA consisted of primary GI carcinoids, matched normal mucosa and peritoneal fibrotic material and was represented by 2 cores/case. Complete clinical details including fibrosis were known for all patients. Clinically significant fibrosis was determined at surgery, and all samples were examined by a pathologist (RLC) to histologically confirm fibrosis.
Serum: Twenty-nine subjects (median age [range] = 42 years [20-83]; M:F = 17:12) attending the Neuroendocrine Referral, Oncology and Surgery outpatient clinics at Yale University School of Medicine were recruited for serum analysis. These included 29 patients with GI carcinoids: SI EC cell carcinoid tumors (n = 16), gastric ECL cell carcinoids (n = 7), and six other GI carcinoids [rectal: n = 2, parotid: n = 1, appendiceal: n = 2, duodenal: n = 1]. Serum samples from ten age-, sex-matched control subjects were also collected.
Quantitative RT-PCR: Total RNA was isolated from frozen carcinoid tumor tissue (n = 10) and normal mucosa (n = 9) with TRIzol reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s guidelines. RNA was dissolved in DEPC water, measured spectrophotometrically and an aliquot analyzed on a denaturing gel using electrophoresis to check the quality of RNA isolated.
CTGF and TGFβ1 message were quantitatively measured in the ten tumor and nine control samples as described[21,22]. Briefly, Q RT-PCR was performed using the ABI 7900 Sequence Detection System. Total RNA from each sample was subjected to reverse transcription using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). 2 μg of total RNA in 50 μL of water was mixed with 50 μL of 2X RT mix containing Reverse Transcription Buffer, dNTPs, random primers and Multiscribe Reverse Transcriptase. RT reaction was carried out in a thermal cycler for 10 min at 25°C followed by 120 min at 37°C. Real time PCR analysis was performed in triplicate[21,22]. cDNA in 7.2 μL of water was mixed with 0.8 μL of 20 × Assays-on-Demand primer (CTGF = Hs00170014, TGFβ1 = Hs00171257, GAPDH = Hs99999905) and probe mix, 8 μL of 2 × TaqMan Universal Master mix in a 384 well optical reaction plate. The following PCR conditions were used: 50°C for 2 min, then 95°C for 10 min, followed by 40 cycles at 95°C/0.15 min and 60°C/1 min. A standard curve was generated for each gene using cDNA obtained by pooling equal amounts from each sample (n = 19). The expression level of target genes was normalized to internal GAPDH. Data was analyzed using Microsoft Excel and calculated using the relative standard curve method (ABI, User Bulletin #2).
Immunohistochemistry: Serial sections (5 μm) encompassing SI carcinoid tumors or fibrotic tissues were deparaffinized in xylene and rehydrated in graded alcohols. For antigen retrieval purposes, sections were immersed in citrate buffer (10 mM sodium citrate, pH 6.0), and subjected to 1 × 10 min high temperature-high pressure treatment followed by treatment with 0.3% H2O2 in methanol for 30 min at 37°C to inactivate endogenous peroxidase. In some studies, sections were incubated with goat antiserum to CTGF (1:250) or TGFβ1 (1:1000) (both from Santa Cruz Biotechnology, Santa Cruz, CA) diluted in Tris-buffered saline containing BSA and a monoclonal antibody against CgA (0.5 μg/mL) or serotonin (2 μg/mL) (both from DAKO, Carpinteria CA) for 24 hr at 4°C and then with Alexa 488-labeled anti-mouse IgG (1:100 dilution) for 1 hr at RT. Donkey anti-goat antibody conjugated to a horseradish peroxidase-decorated dextran polymer backbone (Envision; DAKO Corp, Carpinteria, CA) was used as a secondary reagent. HRP-amplification was performed. CTGF or TGFβ1 was visualized with a fluorescent chromogen (Cy-5-tyramide; NEN Life Science Products, Boston, MA). Dual-positive cells (CTGF + serotonin or CTGF + CgA) were counted in a minimum of 5-well orientated sections and expressed as a percentage. In other studies, fibrotic areas from the peritoneum of patients with SI carcinoid tumors were stained with mouse anti-α-smooth muscle actin (1:1000) or desmin (1:1000, both DAKO), goat anti-vimentin (1:1000), collagen III (1:1000) or CTGF (1:250). Stromal (myofibroblast) cells were separable from tumor cells that were identified by the use of a fluorescently tagged anticytokeratin antibody cocktail (AE1/AE3; DAKO Corp). Nuclei were visualized by 4’, 6-diamidino-2-phenylindole (DAPI 10 mg/mL). Localization of expression of products was used to determine whether stromal (non-cytokeratin staining) or tumor cells expressed these products.
Intestinal stellate cell culture and analysis: Stellate cells were isolated using a modification of the method by Bachem et al[15]. Briefly, cells were isolated from the fibrotic tumor specimen (hand dissected, digested in collagenase (0.25 mg/mL)/DNAse (100 U/mL) solution for 60 min at 37°C under constant aeration) and were cultured on 10 cm2 uncoated culture wells in 10% fetal calf serum in a 1:1 (vol/vol) mixture of DMEM and Ham’s F12 medium supplemented with 2% L-glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 1% amphotericin. Twenty-four hours after seeding, the culture medium was changed and the myofibroblasts remained attached to the plastic. After reaching confluence, cells were subcultured by trypsinization using a 0.025% trypsin solution containing 0.01% EDTA in PBS. For immunofluorescence microscopy, cells were seeded on 1 cm2 glass coverslips in six-well (10 cm2/well; 2 mL medium) plates (2-3 glass coverslips per well). Phase-contrast microscopy was used to identify the translucent fat droplets in the cytoplasm and stellate-like morphology that typifies stellate cells[15]. These studies were undertaken within the first 3-d as culturing cells results in a transdifferentiation from a vitamin A-storing phenotype to a myofibroblastic phenotype[15]. For immunocytological characterization, cells cultured on uncoated glass coverslips were fixed for 30 min in -20°C acetone and air-dried. Coverslips were preincubated for 15 min in TBS (pH 7.4) with 3% bovine serum albumin and 0.3% hydrogen peroxide. Incubations with the primary antibody (mouse monoclonal: α-smooth muscle actin 1:1000) was performed at room temperature in a humidified chamber for 1 h. Non-specific staining was controlled by omitting the primary antibody and including mouse, non-immune serum at the same dilution as used for the specific primary antibody. After rinsing (three times for 5 min with TBS/Tween-0.5%), the second antibody (HRP goat anti-mouse, diluted 1:100) was added and incubated for 1 h at room temperature. Cy5-labelled tyramide (TSA; NEN Life Science Products, Boston, MA) was used with DAPI (10 mg/mL) to stain nuclei and cells observed with a fluorescence microscope. For RNA studies, cultured cells were stimulated with TGFβ1 (10-7 M) for 24 h. Thereafter, RNA was isolated and Q RT-PCR performed as described above to quantitatively measure TGFβ1-stimulated CTGF message.
AQUA Analysis of CTGF and TGFβ1 in the carcinoid TMA: Tissue microarray slides were stained as described[21,23]. Antigen retrieval and immunostaining for CTGF, TGFβ1 (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA), cytokeratin and nuclei were as above. Protein expression (CTGF or TGFβ1) was determined using an automated tissue microarray reader. Automated image acquisition and analysis using AQUA has been described previously[21,23]. In brief, monochromatic, high-resolution (1024 × 1024 pixel; 0.5-μm) images were obtained of each histospot. Areas of tumor separate from stromal elements were distinguished by creating a mask from the cytokeratin signal. Coalescence of cytokeratin at the cell surface localized the cell membranes, and DAPI was used to identify nuclei. The Cy-5 signal from the membrane area of tumor cells was scored on a scale of 0-255 and expressed as signal intensity divided by the membrane area. Histospots containing < 10% tumor, as assessed by mask area (automated), were excluded from further analysis. Previous studies have demonstrated that the staining from a single histospot provides a sufficiently representative sample for analysis[24].
CTGF serum ELISA: Serum CTGF-W (whole molecule) and CTGF-N (N-terminal fragment) were assayed by two separate sandwich enzyme-linked immunosorbent assays (ELISA). The CTGF-W ELISA uses a capture mAB reactive to the amino terminus of CTGF, and detects the bound CTGF-W with an alkaline phosphatase labeled mAb reactive to the carboxyl- terminal region of CTGF. A second ELISA uses two non-cross blocking monoclonal antibodies reacting to distinct NH2-terminal epitopes of CTGF. This assay detected both CTGF-W and CTGF N fragment, so-called CTGF N + W, as described previously[25]. CTGF-N is a value calculated by subtracting CTGF-W from the CTGF N + W level measured by the second assay. Standards for both assays were made from purified full-length CTGF and expressed in nanograms per milliliter. The intra- and interassay coefficient of variation was 5 and 15%, respectively, for both ELISA tests. Data on CTGF-W is presented in this study.
Results are expressed as mean ± SE; n indicates the number of patients in each study group. Statistical significance was calculated by the Student’s test for unpaired values or non-parametric statistics as appropriate. On the TMA, the unpaired 2-tailed Student’s t-test was used to identify statistically significant differences in fibrotic protein expression between different patient groups (patients with clinical evidence of fibrosis versus non-fibrosis, fibrosis versus gastric carcinoid).
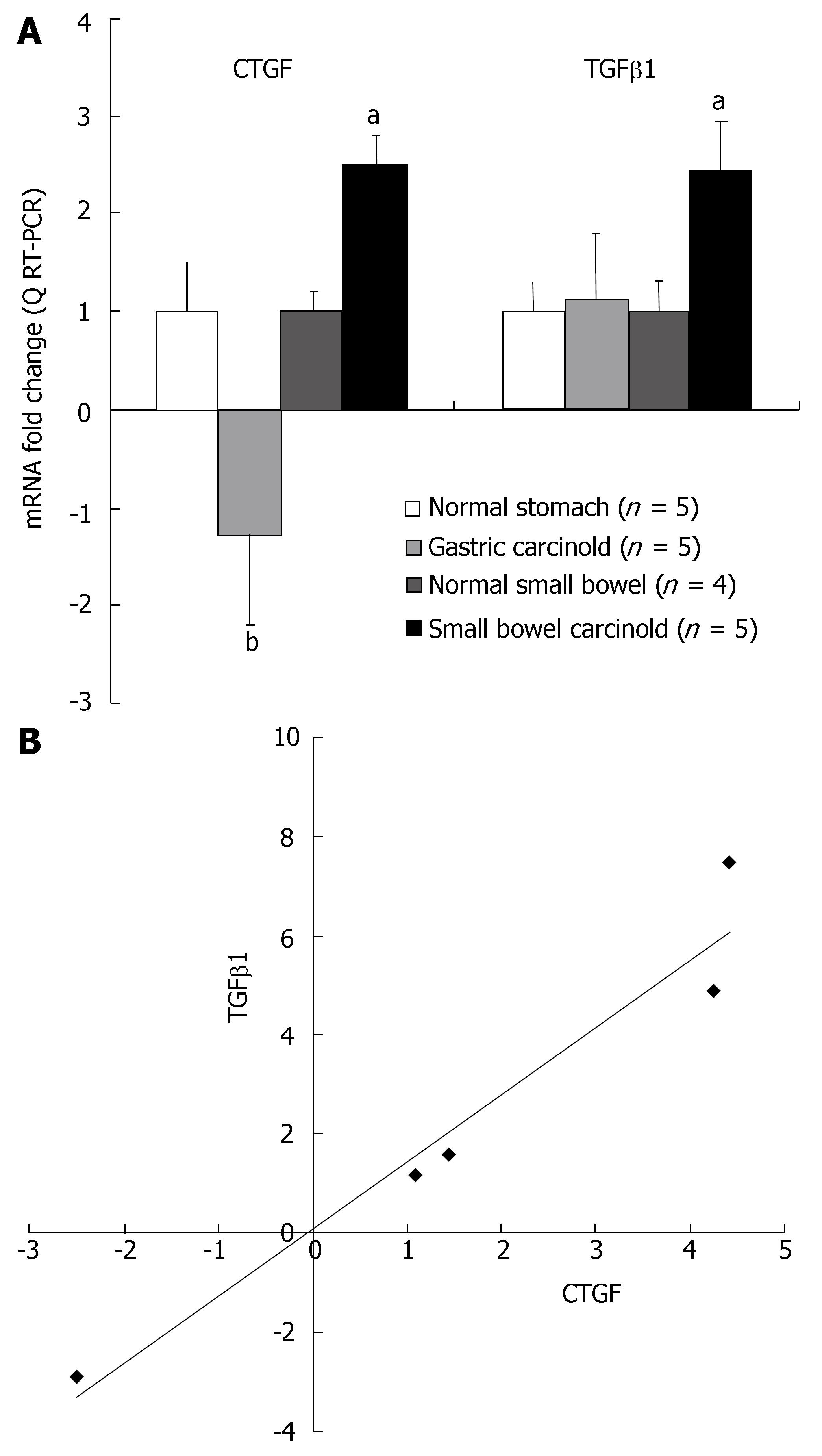
Q RT-PCR analysis was undertaken using Assays on Demand (Applied Biosystems) on the RNA isolated from SI EC cell carcinoid tumors (fibrosis associated) (n = 5); gastric ECL cell tumors (little fibrosis) (n = 5); normal SI mucosal samples (n = 4) and normal gastric mucosa (n = 5) to quantitatively measure the levels of CTGF and TGFβ1 mRNA expression in these two different tumor types. Transcript levels of both CTGF and TGFβ1 were significantly elevated in the five SI carcinoid tumor samples (P < 0.05 vs normal mucosa) (Figure 1A). In contrast, TGFβ1 message was not different (+ 1.13-fold) in gastric carcinoid tumor samples compared to normal, and message levels of CTGF were significantly decreased (-1.3-fold; P < 0.01) compared to SI carcinoid tumors (Figure 1A). There was a good correlation (R2 = 0.95) between CTGF and TGFβ1 message levels in the SI carcinoid tumor samples demonstrating that transcription of these growth factors was tightly associated in this tumor tissue (Figure 1B). No relationship was noted between TGFβ1 mRNA levels and CTGF mRNA levels in gastric carcinoids (R2 = 0.01). These results demonstrate while both gastric and SI carcinoid tumors express mRNA for TGFβ1, CTGF mRNA is over-expressed only in SI carcinoid tumors. CTGF and TGFβ1 transcript levels are associated in SI carcinoid tumors.
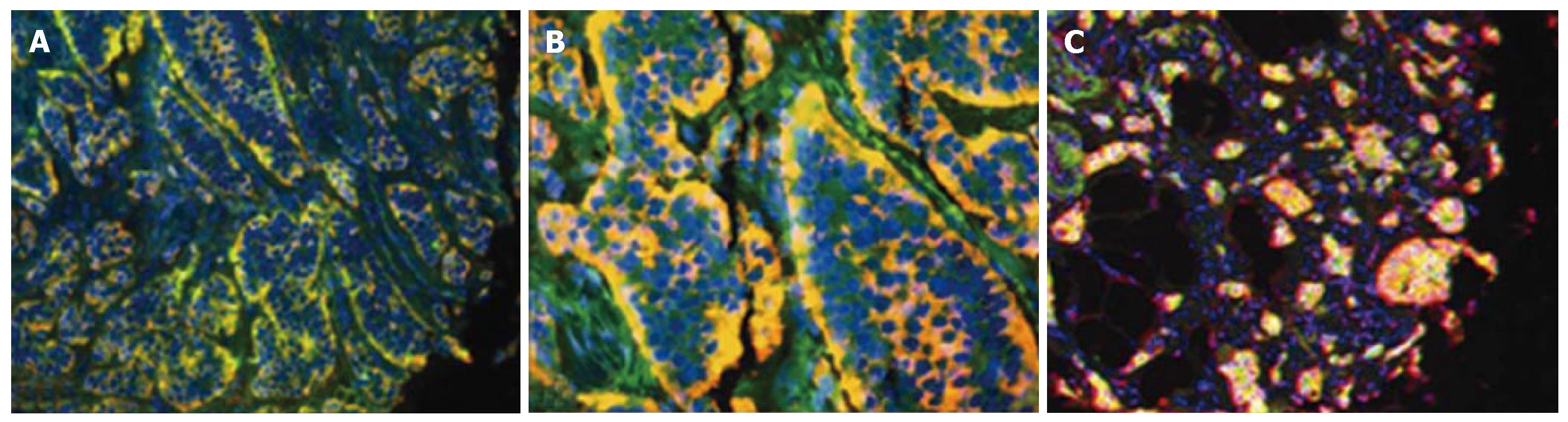
CTGF and TGFβ1 in tumor samples: CTGF was localized in the cytoplasm of SI carcinoid tumor cells (Figure 2). Co-staining with anti-CgA (Figure 2A) or anti-serotonin (Figure 2B) antibodies demonstrated a significant co-localization with CTGF and either antibody (80 ± 12% and 93 ± 6% respectively) in tumor mucosa. Like CTGF, TGFβ1 was cytoplasmic and was present in > 75% of tumor cells (Figure 2C). These results demonstrate that TGFβ1 and CTGF expression are characteristic features of SI EC cell carcinoid tumors.
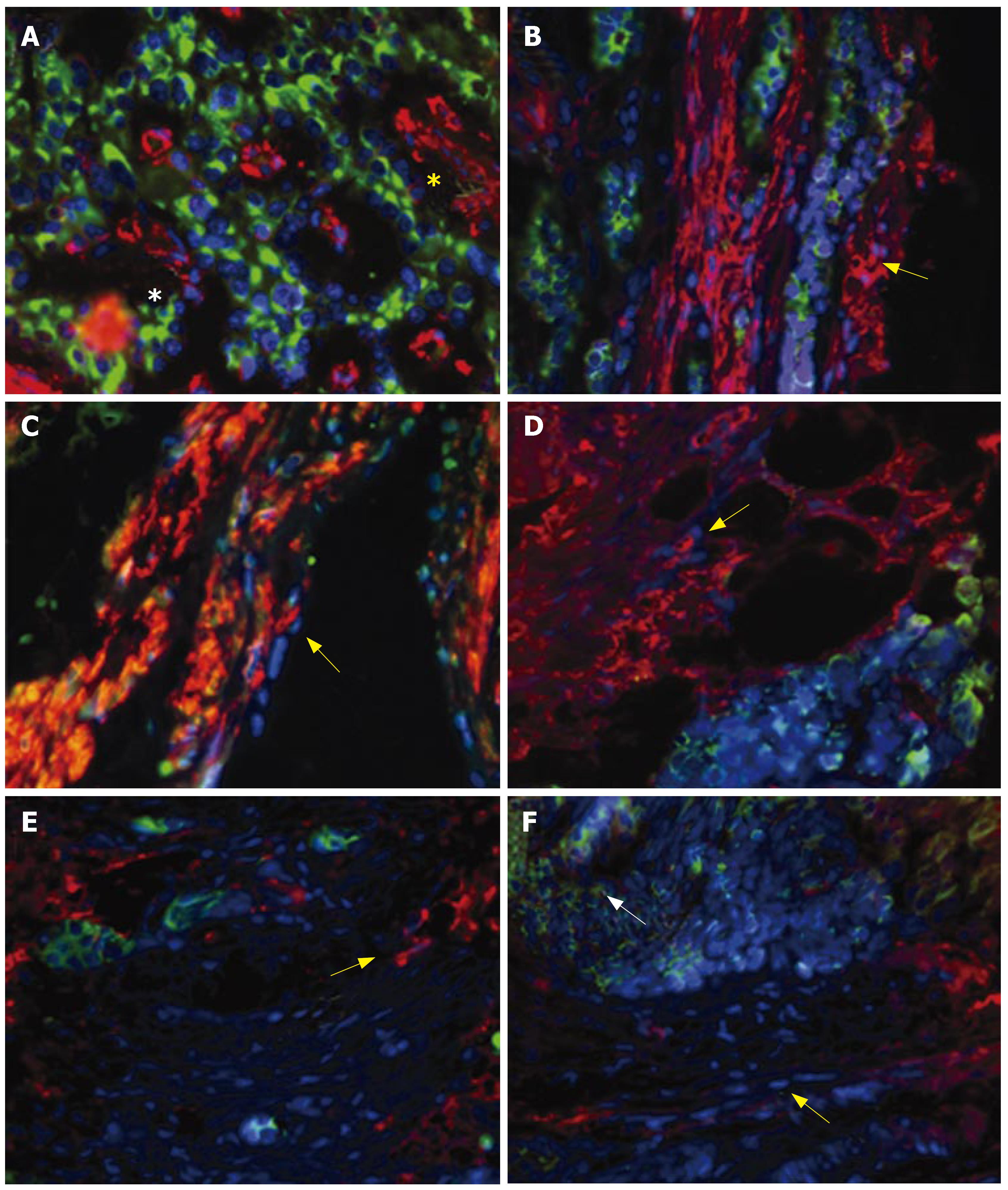
Matrix production in fibrosis:α-smooth muscle actin-positive cells were identified interspersed with carcinoid tumor cells in areas of fibrosis (Figure 3A). α-smooth muscle actin is a marker for activated myofibroblasts (or stellate cells) and indicates that, as for the pancreas, stellate cells are present in peritoneal fibrotic material associated with SI carcinoid tumor mesenteric invasion[15]. Vimentin, desmin and collagen-III positivity was identified with stellate cells (Figure 3B-D). These are markers of a TGFβ1-mediated stellate-cell driven fibrosis[15,19,26], and indicate that this response occurs in SI carcinoid tumors. CTGF was present in both tumor cells and stellate cells (Figure 3E and F), consistent with the expression of this fibrotic mediator in both cell types.
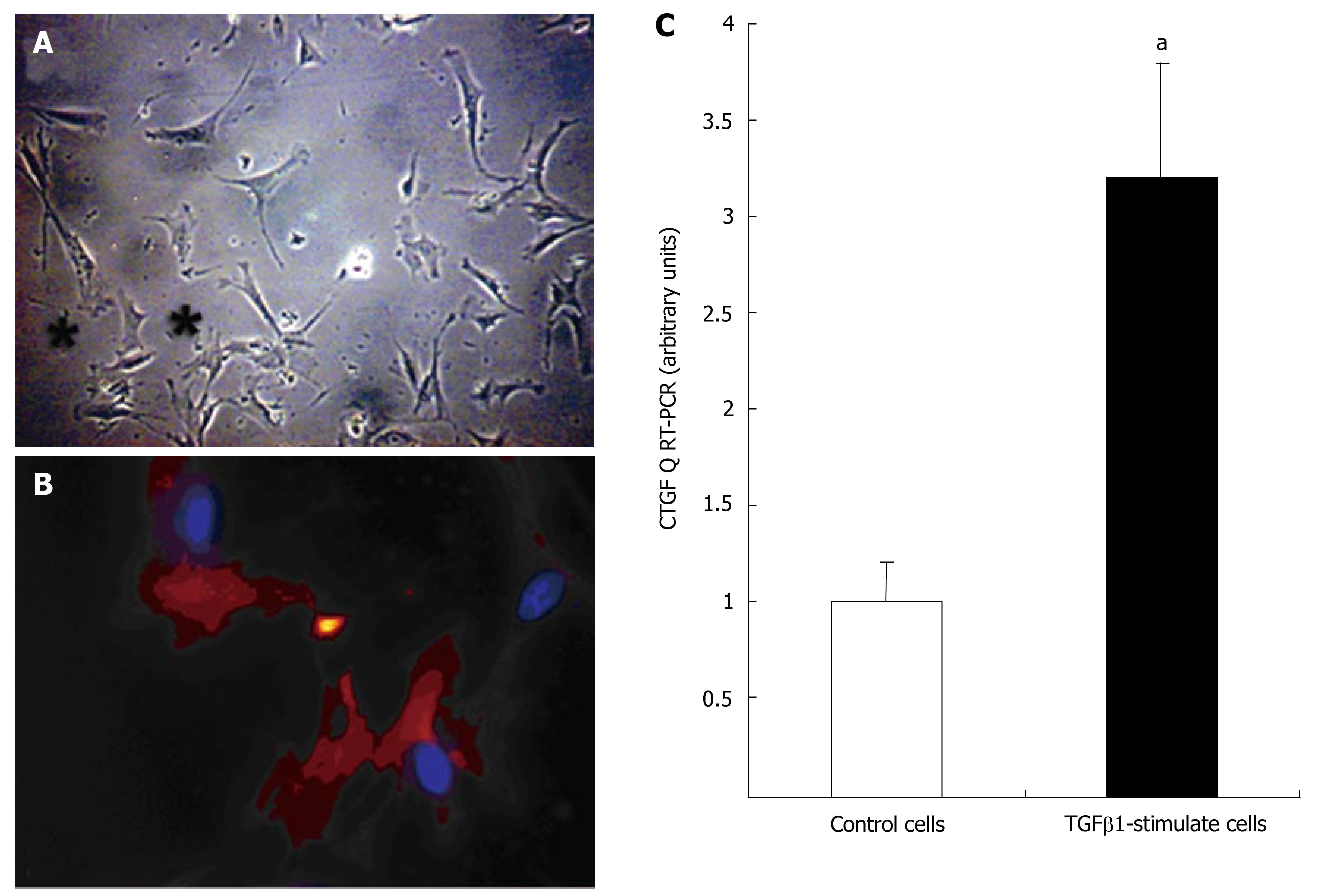
Myofibroblasts from SI carcinoid tumor fibrotic surgical tissue were cultured on plastic as described. Cells in primary cultures flattened and developed long, cytoplasmic extensions. During the 5-7 d in culture, cells developed the typical stellate shape (Figure 4A) and became positive (100%) for α-smooth muscle actin-α marker of myofibroblasts (Figure 4B). This is the classical stellate cell (myofibroblast) activation pathway[15,19]. Stimulating the cells with TGFβ1 (10-7 mol/L) for 24 h significantly increased CTGF mRNA expression (3.2 ± 0.7, P < 0.05 vs un-stimulated cells) (Figure 4C).
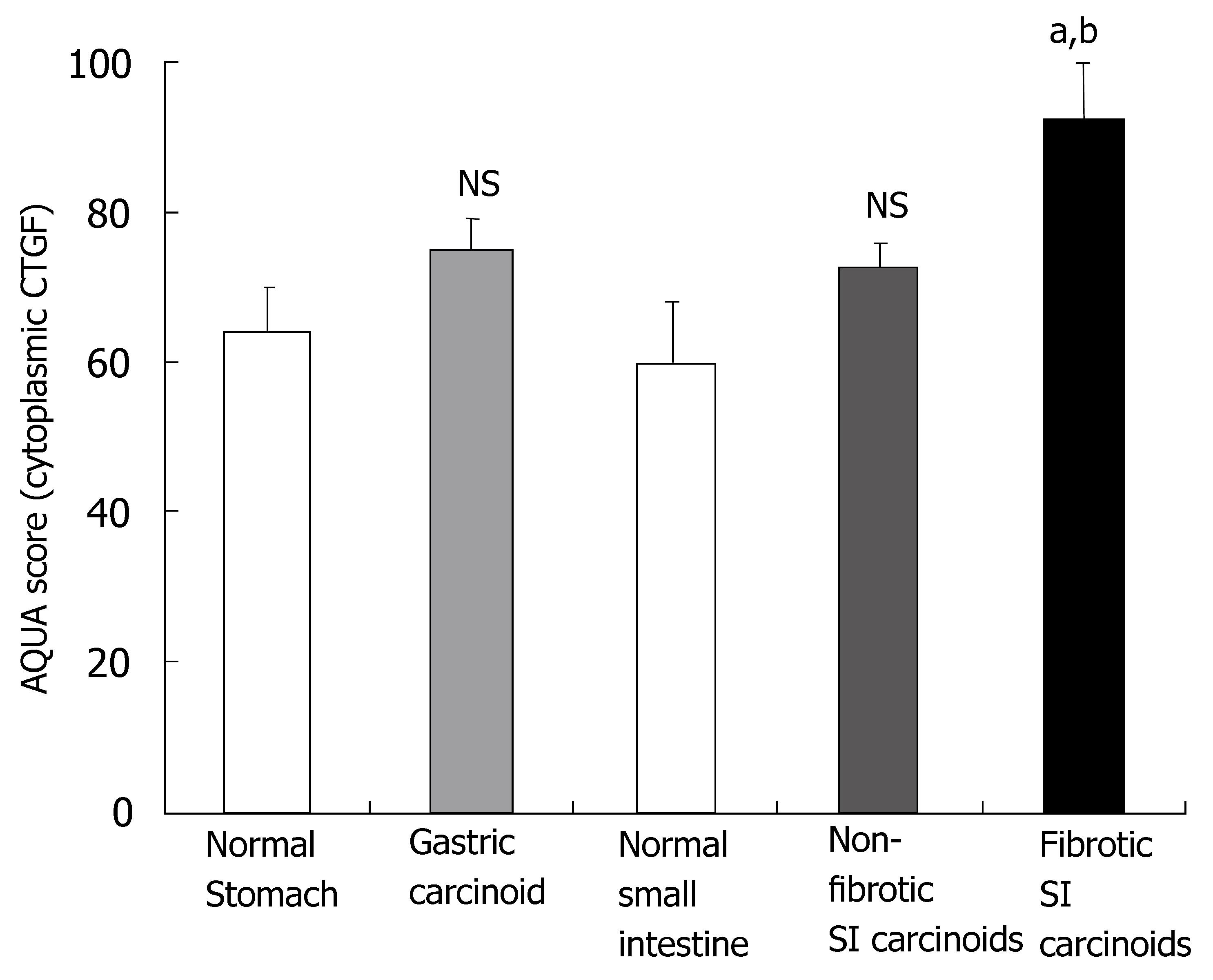
An examination of the CTGF-stained histospots from the 36 patients with SI carcinoid tumors demonstrated that CTGF expression levels ranged from: AQUA score: 49.7-186.3. Higher levels of CTGF staining (AQUA score: 92.5 ± 8.2; P = 0.017) were identified in the fifteen SI carcinoid tumor patients with clinical (surgical) and histologically documented evidence of peritoneal fibrosis compared to the twenty-one patients (AQUA score: 72.7 ± 3.2) with no evidence of fibrotic disease (Figure 5). CTGF levels in non-tumor, non-fibrotic normal SI mucosal tissue were significantly lower (59 ± 4, P < 0.005) than in patients with clinically and histologically documented fibrotic disease.
An examination of the CTGF-stained histospots from the seven patients with gastric carcinoids assessed by AQUA demonstrated that expression levels were not elevated in these patients compared to normal matched gastric mucosa (64 ± 3 vs 72 ± 3) but were significantly lower than in SI carcinoid tumors associated with fibrosis (P < 0.03).
An examination of the TGFβ1-stained histospots from patients with SI carcinoid tumors demonstrated that although TGFβ1 expression levels were elevated in patients with documented fibrosis (AQUA score: 90.6 ± 4.4) compared to the patients with no evidence of fibrotic disease (AQUA score: 82.7 ± 4.0) this did not reach statistical significance (P = 0.08). TGFβ1 levels were, however, lower in the matched normal SI mucosal samples (65 ± 4, P < 0.05 versus fibrotic tumor samples). In the gastric mucosa, expression levels were not elevated in patients with gastric carcinoids compared to normal matched mucosa (61 ± 5 vs 64 ± 3) but, as for CTGF, values in these non-fibrotic samples were significantly lower than in SI carcinoid tumors associated with fibrosis (P < 0.03).
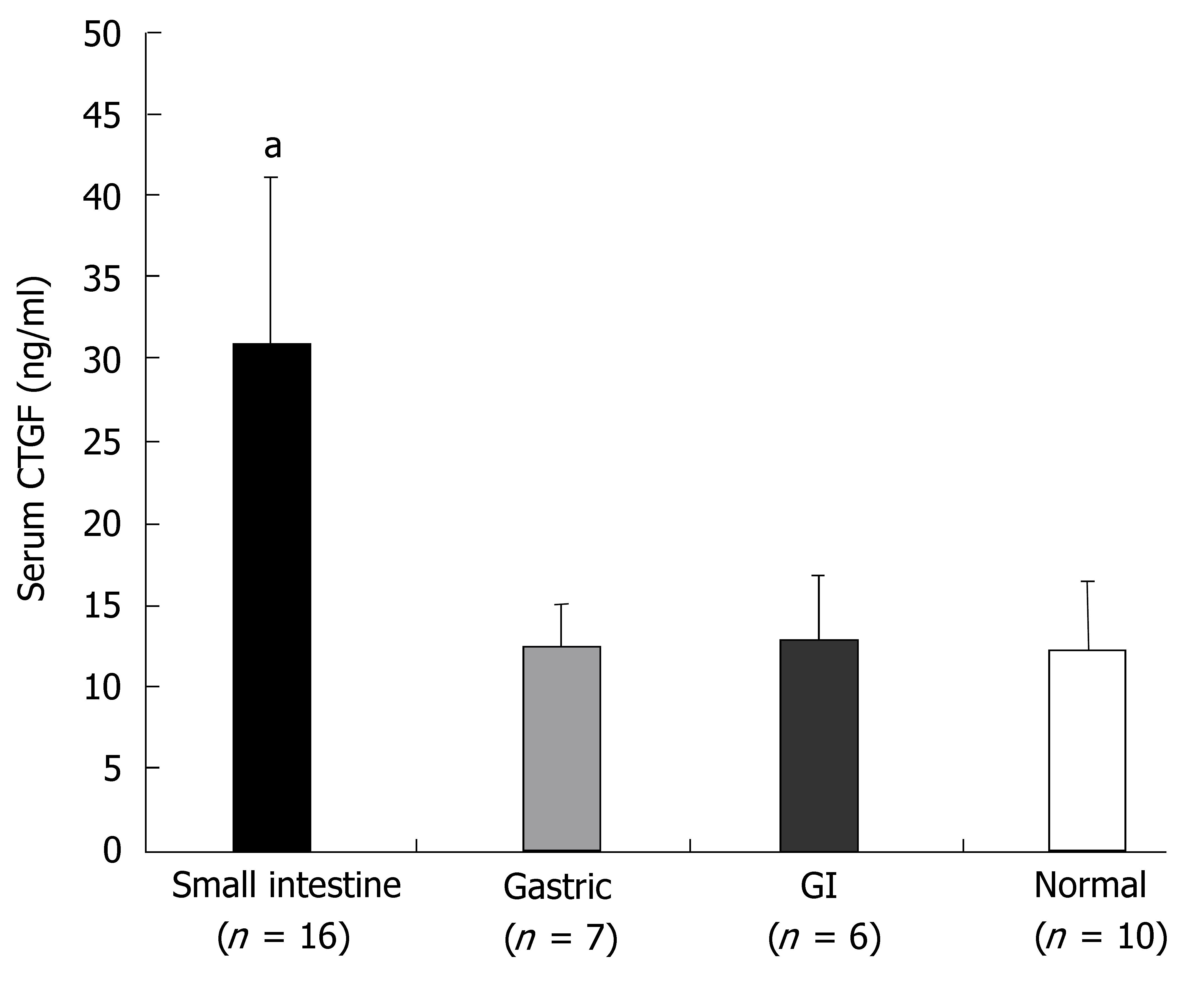
Serum levels of CTGF ranged from 7.2-171 ng/mL. Significantly higher serum CTGF levels were found in patients with SI carcinoid tumors (31.0 ± 10) than in patients with ECL cell carcinoids (12.5 ± 4.9, P < 0.03), other GI carcinoids (12.9 ± 0.6, P < 0.04) and control patients (12.4 ± 4, P < 0.02) (Figure 6). A comparison of serum CTGF levels with tissue levels of CTGF (AQUA scores) (where available) identified a strong correlation between these two measurements (R2 = 0.91, P < 0.005, n = 9).
In the current study, we present data in support of our hypothesis that fibrosis is associated with invasion of the mesentery by SI carcinoid tumor cells and is a consequence of the secretory activity of these cells. In addition we have demonstrated that the mechanism may be due to CTGF production, and TGFβ related events that activate an intestinal stellate (myofibroblastic) cell resulting in a local desmoplastic response. The latter is responsible for the clinical consequences of mesenteric fibrosis and adhesive obstruction noted in SI carcinoid tumors.
In our studies, Q RT-PCR demonstrated that all samples from patients with SI carcinoid tumors had elevated CTGF message levels (+ 1.1 to + 4.4-fold). In contrast, non-fibrotic gastric ECL cell carcinoids had significantly decreased CTGF levels. This analysis demonstrates that CTGF was quantitatively over-expressed in SI tumors. Message levels for TGFβ1 were elevated in SI carcinoid tumor samples but not in gastric samples. These results indicate that CTGF and TGFβ1 are potentially functionally related in the tumor EC cell but not in the ECL cell. We have previously reported that type I gastric (ECL cell) carcinoids (with no evidence of fibrosis) failed to express detectable levels of CTGF message by standard RT-PCR[27]. These results suggest that CTGF message produced by a transformed neuroendocrine cell (the SI EC cell) is associated with fibrosis.
Immunohistochemistry demonstrated that the majority (> 75%) of SI carcinoid tumor cells expressed CTGF. In normal mucosa, CTGF immunostaining was restricted to the basal third of the SI crypts with either CgA or serotonin-positive cells. Approximately one-third of serotonin-expressing (EC) cells were CTGF-positive (data not shown). It is likely that the remainder of the CTGF-staining cells are myofibroblasts in the crypts. CTGF-positive myofibroblasts have previously been demonstrated in the rectum[28].
Carcinoid tumor cells also express TGFβ1, and presumably this growth factor is secreted by these cells during mesenteric invasion. This was previously noted by Chaudhry et al[20] who reported that stromal cells expressed the TGFβ receptor. This suggests a mechanism by which tumor cells can interact with stromal cells and influence their function. Our immunohistochemical analysis demonstrated that stromal cells in areas of mesenteric fibrosis were α-smooth muscle actin positive. α-smooth muscle actin is a marker for activated myofibroblasts (or stellate cells[15,19]) and indicates that fibrosis-induction in the small intestine is associated with a stellate cell phenotype. This is a typical phenotype of both pancreatic- and hepatic-associated fibrosis[17,19], and suggests this may be an archetypical GI fibrotic phenomenon. This postulate is supported by evidence that vimentin, desmin and collagen-III, all markers of a stellate-cell driven fibrosis, were present in SI fibrosis.
In order to confirm whether stellate cells were present in this tissue and played a role in fibrosis, we isolated and characterized a cell type from a patient with SI carcinoid tumor fibrosis that exhibited the hallmarks of a stellate cell[15]. During primary culture, this cell flattened, initially developed long, cytoplasmic extensions, and subsequently, the typical stellate shape of activated myofibroblasts. The presence of α-smooth muscle actin staining confirmed the stellate cell phenotype. Addition of TGFβ1 resulted in activation of CTGF message and demonstrated the cell type to be functionally responsive to this growth factor. These functional data, together with the immunohistochemical evidence of activated intestinal stellate cells in situ, strongly suggest that carcinoid-induced fibrosis is a stellate-cell induced phenomenon. It is possible that the “intestinal stellate” cell could be derived from precursor cells in blood stream and there is some evidence that bone marrow-derived cells can migrate into the SI[29]. A study of hepatic stellate cells, however, conclusively identified that these cells were not derived from bone marrow derived fibrocytes[30]. The latter did not stain for a-smooth muscle actin or desmin and were considered a separate population within the liver. This, as well as our immunohistochemical results strongly suggests the presence of an endogenous intestinal stellate cell population.
Having established that mesenteric fibrosis was associated with elevated CTGF and TGFβ1 in SI carcinoid tumors and identified a mesenteric target cell (intestinal stellate cell), we next used TMA analysis to both quantitate the protein expression as well as the cellular source of CTGF and TGFβ1 and statistically determine whether these proteins were related to clinically and histologically documented evidence of fibrosis. Our results demonstrated that TGFβ1 levels were elevated in patients with fibrosis, and were significantly increased compared to normal SI mucosa and to gastric carcinoids. The difference in protein expression between fibrotic SI carcinoid tumors and non-fibrotic gastric carcinoid samples identified on the TMA further supports a role for TGFβ1 in the etiology of this fibrosis. The role of CTGF was confirmed by the unambiguous relationship between increased expression of CTGF protein in primary SI carcinoid tumors and fibrosis. It is of interest to note that five patients who initially had exhibited elevated CTGF AQUA scores (87 ± 5) on the TMA subsequently developed fibrosis.
In order to identify a clinically useful tool to recognize patients at risk for fibrosis, we sought to measure CTGF in serum. Secreted CTGF protein could be identified in patient serum and was elevated in patients with SI carcinoid tumors compared to patients with gastric ECL cell carcinoids. Serum levels of CTGF from the latter patient group were similar to values in control subjects as might be predicted given that the gastric carcinoids are not associated with carcinoid fibrosis. The highest levels of serum CTGF in this study were identified in two patients with SI carcinoid tumors who also had the typical carcinoid “flushing” symptoms consistent with disseminated disease. This suggests this protein is identifiable in serum and can discriminate SI from gastric carcinoids. Prospective longitudinal studies in patients with and without fibrosis are needed to determine whether plasma levels have clinical significance in the detection, or prediction of peritoneal or cardiac fibrosis.
In conclusion, SI carcinoid tumors over-express CTGF and TGFβ1 mRNA and synthesize CTGF and TGFβ1 protein which are significantly elevated in patients with clinically documented fibrosis. In addition, SI carcinoid tumors secrete CTGF, which is readily detectable in the serum. We have also immunohistochemically identified and biochemically characterized intestinal stellate cells from mesenteric fibrosis. These cells respond to TGFβ1 with CTGF mRNA transcription. In addition, matrix production in SI carcinoid tumor fibrosis was similar to that identified in other stellate cell-driven reactions (e.g., liver or pancreas)[15,17,19]. We postulate that intestinal stellate cells are the target cells that are activated by profibrotic mediators (TGFβ1 and CTGF) synthesized and secreted by invasive SI carcinoid tumor cells. Furthermore, once activated, these stellate cells may auto-regulate the fibrotic phenotype (by production of CTGF). The detection of blood levels of CTGF may ultimately provide a diagnostic opportunity to predict the development of fibrosis and pre-empt its local and systemic complications.
Supported in part by the Bruggeman Medical Foundation
S- Editor Liu Y L- Editor Li M E-Editor Li JL
| 1. | Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1852] [Article Influence: 84.2] [Reference Citation Analysis (1)] |
| 2. | Modlin IM, Champaneria MC, Chan AK, Kidd M. A three-decade analysis of 3,911 small intestinal neuroendocrine tumors: the rapid pace of no progress. Am J Gastroenterol. 2007;102:1464-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | McNeal JE. Mechanism of obstruction in carcinoid tumors of the small intestine. Am J Clin Pathol. 1971;56:452-458. [PubMed] |
| 4. | Harvey JN, Denyer ME, DaCosta P. Intestinal infarction caused by carcinoid associated elastic vascular sclerosis: early presentation of a small ileal carcinoid tumour. Gut. 1989;30:691-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Levy AD, Rimola J, Mehrotra AK, Sobin LH. From the archives of the AFIP: benign fibrous tumors and tumorlike lesions of the mesentery: radiologic-pathologic correlation. Radiographics. 2006;26:245-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Modlin IM, Shapiro MD, Kidd M. Carcinoid tumors and fibrosis: an association with no explanation. Am J Gastroenterol. 2004;99:2466-2478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Moertel CG, Sauer WG, Dockerty MB, Life history of the carcinoid tumor of the small intestine. Cancer. 1961;14:901-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Modlin IM, Lye KD, Kidd M. A 50-year analysis of 562 gastric carcinoids: small tumor or larger problem? Am J Gastroenterol. 2004;99:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1854] [Article Influence: 88.3] [Reference Citation Analysis (1)] |
| 10. | Leask A, Holmes A, Abraham DJ. Connective tissue growth factor: a new and important player in the pathogenesis of fibrosis. Curr Rheumatol Rep. 2002;4:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Moussad EE, Brigstock DR. Connective tissue growth factor: what's in a name? Mol Genet Metab. 2000;71:276-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 390] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 12. | Robson MC. Proliferative scarring. Surg Clin North Am. 2003;83:557-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996;7:469-480. [PubMed] |
| 14. | Ignotz RA, Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337-4345. [PubMed] |
| 15. | Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grünert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 798] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 16. | Wenger C, Ellenrieder V, Alber B, Lacher U, Menke A, Hameister H, Wilda M, Iwamura T, Beger HG, Adler G. Expression and differential regulation of connective tissue growth factor in pancreatic cancer cells. Oncogene. 1999;18:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Jaskiewicz K, Nalecz A, Rzepko R, Sledzinski Z. Immunocytes and activated stellate cells in pancreatic fibrogenesis. Pancreas. 2003;26:239-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Schneider E, Schmid-Kotsas A, Zhao J, Weidenbach H, Schmid RM, Menke A, Adler G, Waltenberger J, Grünert A, Bachem MG. Identification of mediators stimulating proliferation and matrix synthesis of rat pancreatic stellate cells. Am J Physiol Cell Physiol. 2001;281:C532-C543. [PubMed] |
| 19. | Wells RG, Crawford JM. Pancreatic stellate cells: the new stars of chronic pancreatitis? Gastroenterology. 1998;115:491-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Chaudhry A, Oberg K, Gobl A, Heldin CH, Funa K. Expression of transforming growth factors beta 1, beta 2, beta 3 in neuroendocrine tumors of the digestive system. Anticancer Res. 1994;14:2085-2091. [PubMed] |
| 21. | Kidd M, Eick G, Shapiro MD, Camp RL, Mane SM, Modlin IM. Microsatellite instability and gene mutations in transforming growth factor-beta type II receptor are absent in small bowel carcinoid tumors. Cancer. 2005;103:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Kidd M, Modlin IM, Mane SM, Camp RL, Eick GN, Latich I, Zikusoka MN. Utility of molecular genetic signatures in the delineation of gastric neoplasia. Cancer. 2006;106:1480-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med. 2002;8:1323-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 611] [Cited by in RCA: 619] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 24. | Camp RL, Charette LA, Rimm DL. Validation of tissue microarray technology in breast carcinoma. Lab Invest. 2000;80:1943-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 569] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 25. | Gilbert RE, Akdeniz A, Weitz S, Usinger WR, Molineaux C, Jones SE, Langham RG, Jerums G. Urinary connective tissue growth factor excretion in patients with type 1 diabetes and nephropathy. Diabetes Care. 2003;26:2632-2636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Arnaud A, Fontana L, Angulo AJ, Gil A, López-Pedrosa JM. Proliferation, functionality, and extracellular matrix production of hepatocytes and a liver stellate cell line: a comparison between single cultures and cocultures. Dig Dis Sci. 2003;48:1406-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Kidd M, Modlin I, Lye K, Usinger W, Murren J. ASCO 2004 Gastrointestinal Cancers Symposium Connective tissue growth factor (CTGF) is over-expressed in ileal carcinoids. 2004;. |
| 28. | Gervaz P, Hennig R, Buechler M, Soravia C, Brigstock DR, Morel P, Egger JF, Friess H. Long-term expression of fibrogenic cytokines in radiation-induced damage to the internal anal sphincter. Swiss Surg. 2003;9:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Nakao A, Toyokawa H, Kimizuka K, Nalesnik MA, Nozaki I, Bailey RJ, Demetris AJ, Starzl TE, Murase N. Simultaneous bone marrow and intestine transplantation promotes marrow-derived hematopoietic stem cell engraftment and chimerism. Blood. 2006;108:1413-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, Brenner DA. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 366] [Article Influence: 19.3] [Reference Citation Analysis (0)] |