Published online Oct 14, 2007. doi: 10.3748/wjg.v13.i38.5151
Revised: August 1, 2007
Accepted: August 7, 2007
Published online: October 14, 2007
Sirolimus is an immunosuppressant with expanding use in pediatric organ transplantation, dermatology and rheumatology. We report two cases of children who developed asthma like symptoms and were diagnosed with interstitial lung disease, which responded to discontinuation of sirolimus. Pediatricians should be aware about the pulmonary side effects of sirolimus.
- Citation: Gupte G, Mahadevan S, Clarke J, Alton H, Beath S. Sirolimus-related pulmonary toxicity mimicking 'asthma like' symptoms. World J Gastroenterol 2007; 13(38): 5151-5153
- URL: https://www.wjgnet.com/1007-9327/full/v13/i38/5151.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i38.5151
Sirolimus (rapamycin) is a macrolide immunosuppressant with increasing use in various pediatric subspecialities[1,2]. Its dose-related side effects leading to thrombocytopenia and hypercholesterolemia are widely known. Pulmonary toxicity associated with sirolimus therapy has only been recently recognized as a potentially serious complication[3]. Among the twenty-eight children (18 with liver transplant and 10 with small bowel transplant) treated with sirolimus in our center, two developed pulmonary complications which are described in this case report.
A 2-year-old girl with microvillous inclusion disease underwent combined small bowel and liver transplantation (CSBLTx). She received tacrolimus and prednisolone according to our immunosuppression protocol. She developed severe rejection at the age of 2 years due to poor compliance with medications. Sirolimus (0.1 mg/kg per day) was added to her immunosuppression regime aiming at levels of 8-10 ng/mL for both tacrolimus and sirolimus.
Four months after starting sirolimus treatment, she developed a perforation near the anastomotic site of the intestinal allograft and sirolimus was stopped. She remained generally well in the next year, but in view of deteriorating renal function, and persistent hypomagnesaemia, the tacrolimus dose was halved (target level 3-5 ng/mL) and sirolimus was added (target level 8-10 ng/mL). Two months after commencing sirolimus she acquired adenovirus infection which required mechanical ventilation and reduction of immunosuppression (target level of tacrolimus and sirolimus 3-5 ng/mL). After the reduction of immunosuppression, she showed an uneventful recovery.
During the next 5 mo (now 4 years and 9 mo after transplantation), in which sirolimus was re-introduced, she developed a dry cough and increased respiratory rate with mild subcostal recession. Her clinical examination was otherwise unremarkable. Initially she had no response to bronchodilators for possible asthma. Pulmonary function tests were normal. Her chest X-ray showed some loss of volume in the left lower lobe and coarse interstitial shadowing throughout both lung fields. Immunofluorescence and viral culture of nasopharyngeal aspirates and broncho-alveolar lavage (BAL) including adenovirus were all negative. The BAL fluid grew haemophilus influenzae, which was treated with amoxicillin. Blood cultures for fungi and bacteria were negative, as was PCR for CMV and EBV. A high resolution CT scan of the chest demonstrated fine nodular changes in the interstitial air spaces. A lung biopsy was considered but deferred. Sirolimus-associated pulmonary toxicity was suspected and sirolimus was discontinued. Her respiratory symptoms of cough and dyspnea improved within 3 mo and her chest X-ray changes resolved within 6 mo.
A 9-mo-old boy with Hirschprung disease with total aganglionosis extending proximally to the pylorus underwent CSBLTx. He was commenced on tacrolimus and prednisolone according to our standard immunosuppression protocol. An episode of moderate acute rejection 10 d after transplant was treated with a single dose of steroids and two doses of basiliximab four days apart. Sirolimus (0.14 mg/kg) was commenced 6 wk after transplant. The aim was to maintain tacrolimus and sirolimus levels at 8-10 ng/mL and 6-8 ng/mL, respectively. Five months after commencing sirolimus, he showed features of bone marrow suppression with neutropenia and anemia. A bone marrow aspirate showed myeloid maturation arrest and responded well to weekly injection of granulocyte colony stimulating factor for 3 mo and supportive treatment.
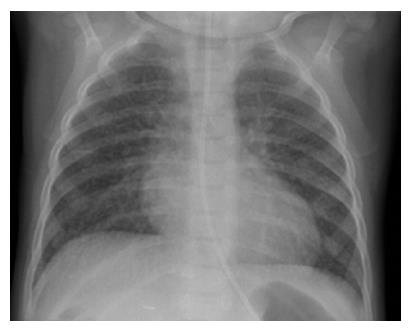
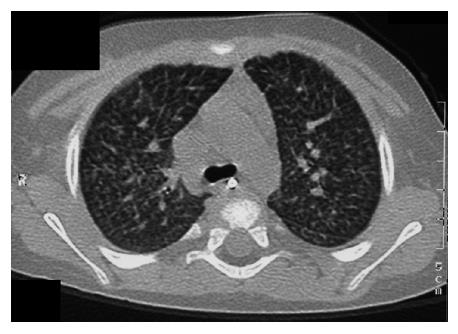
In view of bone marrow suppression, the dose of sirolimus was reduced to maintain a level 4-6 ng/mL. Seven months after introduction of sirolimus, he presented with a persistent dry cough and breathlessness on exertion. Respiratory examination was normal. Chest X-ray showed bilateral interstitial shadowing and nodularity (Figure 1), but overnight pulse oximetry observation was normal. A comprehensive evaluation for post-transplant lymphoproliferative disease (PTLD), including biopsies from upper GI endoscopy, liver biopsy, and ileoscopy was negative. EBV PCR titer was low with a level of 1000 genome copies/mL. Investigations for respiratory infection, including BAL, revealed no infection and special staining for pneumocystis carinii pneumonia, acid-fast bacilli staining and immunochemistry for cytomegalovirus and Epstein-Barr virus were negative. A high resolution CT scan revealed thickening of the interlobular septae with fine nodular changes (Figure 2). A lung biopsy was considered and deferred. Following discontinuation of sirolimus, his symptoms resolved within 4 wk, but radiological changes were not resolved after 18 mo of follow-up.
The resolution of clinical symptoms and improvement in radiological changes after withdrawal of sirolimus in our patients, with the absence of other infectious factors, strongly implicates that sirolimus is the causative factor for the pulmonary changes.
Sirolimus-associated pulmonary toxicity is mostly described in the adult literature and actually represents a spectrum of clinico-pathologic syndromes characterized clinically by dyspnea, cough, fever, fatigue or haemoptysis, and histologically by the presence of organizing pneumonia, interstitial pneumonitis, focal fibrosis or by the presence of alveolar haemorrhage[4]. In the largest report on sirolimus-associated pulmonary toxicity in adult kidney transplant recipients, features of pneumonitis were seen within 6-12 mo after commencing sirolimus therapy[3]. Our patients showed a similar time course .
The children in this report exhibited ‘asthma like’ symptoms with recurrent episodes of dry cough and breathlessness on exertion. In children on immuno-suppressive drugs, the diagnostic challenge is to rule out opportunistic infections. Lung biopsy looking for any histological changes was not performed due to the invasive nature of the procedure and resolution of the symptoms and radiological changes on cessation of sirolimus. A review of drug history did not identify any other medicines in the complex cases, which could give rise to the ‘asthma’ like symptoms.
The management consists of excluding other etiologies, especially opportunistic infections, and discontinuation or dose reduction of sirolimus[3]. As in the previous reports, respiratory symptoms resolved within 2-4 wk after cessation of sirolimus therapy with improvement of CXR changes in 6-18 mo, which is consistent with other case reports[3,5]. We opted to stop the treatment as the long-term outcome of interstitial lung changes in children is not known. It is possible that the degree of reversal of pulmonary symptoms depends on the extent and the chronicity of parenchymal and interstitial changes. The children reported were on low dose steroids as a part of their immunosupression regime and developed interstitial lung disease despite being on steroids.
The exact pathogenic mechanism of sirolimus-induced pulmonary toxicity is not known. Possible mechanisms include idiosyncratic cell-mediated autoimmune response due to the exposure of cryptic antigens and T cell-mediated delayed type hypersensitivity reaction[3,6]. Of the 28 children (10 with small bowel transplant, 18 with liver transplant) treated with sirolimus in the liver unit at BCH, two children with small bowel transplant developed the changes, while none of the children with liver transplants developed this complication. It is entirely possible that the complication may be related to the higher intensity of immunosuppression used in the children with intestinal transplantation. However, a simple dose-dependant toxicity reaction seems less likely since other dose-dependent side effects such as thrombocytopenia or hypercholesterolemia were absent. Morath C et al[7] have documented that an increase in sirolimus levels 3 wk prior to the onset of symptoms and an older age are the risk factors for developing interstitial lung disease. Similar observations could not be made from our two cases.
In conclusion, with the expanding use of sirolimus in children, the appearance of persistent respiratory symptoms, especially cough and dyspnea, should alert the pediatrician to the possibility of sirolimus-associated pulmonary toxicity and the drug may have to be discontinued.
The authors gratefully acknowledge the help from Dr. M Sood (Paediatric Gastroenterologist, Booth Hall Children's Hospital, Manchester) and Dr. S Mitton (Paediatric Gastroenterologist, St George’s Hospital, London) in the shared care of these complex patients.
S- Editor Liu Y L- Editor Wang XL E- Editor Lu W
| 1. | Marsland AM, Griffiths CE. The macrolide immunosuppressants in dermatology: mechanisms of action. Eur J Dermatol. 2002;12:618-622. [PubMed] |
| 2. | Drosos AA. Newer immunosuppressive drugs: their potential role in rheumatoid arthritis therapy. Drugs. 2002;62:891-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Pham PT, Pham PC, Danovitch GM, Ross DJ, Gritsch HA, Kendrick EA, Singer J, Shah T, Wilkinson AH. Sirolimus-associated pulmonary toxicity. Transplantation. 2004;77:1215-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 214] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Singer SJ, Tiernan R, Sullivan EJ. Interstitial pneumonitis associated with sirolimus therapy in renal-transplant recipients. N Engl J Med. 2000;343:1815-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Lennon A, Finan K, FitzGerald MX, McCormick PA. Interstitial pneumonitis associated with sirolimus (rapamycin) therapy after liver transplantation. Transplantation. 2001;72:1166-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Morelon E, Stern M, Israël-Biet D, Correas JM, Danel C, Mamzer-Bruneel MF, Peraldi MN, Kreis H. Characteristics of sirolimus-associated interstitial pneumonitis in renal transplant patients. Transplantation. 2001;72:787-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 211] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Morath C, Schwenger V, Ksoll-Rudek D, Sommerer C, Beimler J, Schmidt J, Zeier M. Four cases of sirolimus-associated interstitial pneumonitis: identification of risk factors. Transplant Proc. 2007;39:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |