Published online Oct 14, 2007. doi: 10.3748/wjg.v13.i38.5043
Revised: August 11, 2007
Accepted: August 26, 2007
Published online: October 14, 2007
Severe acute pancreatitis (SAP) develops in about 25% of patients with acute pancreatitis (AP). Severity of AP is linked to the presence of systemic organ dysfunctions and/or necrotizing pancreatitis pathomorphologically. Risk factors determining independently the outcome of SAP are early multi-organ failure, infection of necrosis and extended necrosis (> 50%). Up to one third of patients with necrotizing pancreatitis develop in the late course infection of necroses. Morbidity of SAP is biphasic, in the first week strongly related to early and persistence of organ or multi-organ dysfunction. Clinical sepsis caused by infected necrosis leading to multi-organ failure syndrome (MOFS) occurs in the later course after the first week. To predict sepsis, MOFS or deaths in the first 48-72 h, the highest predictive accuracy has been objectified for procalcitonin and IL-8; the Sepsis-Related Organ Failure Assessment (SOFA)-score predicts the outcome in the first 48 h, and provides a daily assessment of treatment response with a high positive predictive value. Contrast-enhanced CT provides the highest diagnostic accuracy for necrotizing pancreatitis when performed after the first week of disease. Patients who suffer early organ dysfunctions or at risk of developing a severe disease require early intensive care treatment. Early vigorous intravenous fluid replacement is of foremost importance. The goal is to decrease the hematocrit or restore normal cardiocirculatory functions. Antibiotic prophylaxis has not been shown as an effective preventive treatment. Early enteral feeding is based on a high level of evidence, resulting in a reduction of local and systemic infection. Patients suffering infected necrosis causing clinical sepsis, pancreatic abscess or surgical acute abdomen are candidates for early intervention. Hospital mortality of SAP after interventional or surgical debridement has decreased in high volume centers to below 20%.
- Citation: Beger HG, Rau BM. Severe acute pancreatitis: Clinical course and management. World J Gastroenterol 2007; 13(38): 5043-5051
- URL: https://www.wjgnet.com/1007-9327/full/v13/i38/5043.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i38.5043
Dealing with the clinical course of acute pancreatitis (AP) and the management of severe acute pancreatitis (SAP) are complicated by limited understanding of pathogenesis and multi-causality of the disease, uncertainties to predict outcome and a few effective treatment modalities. AP comprises clinically a mild oedematous-interstitial inflammation, which is a self-limiting disease, and a severe type of AP with a local necrotizing inflammation and systemic complications. Despite the importance of recognizing severe disease early in the course, many patients initially identified as having mild disease, progress to severe pancreatitis over the initial period of disease. Clinical studies and experimental new data have led to considerable progress in understanding the pathophysiological events of the early period of human AP, but the underlying processes leading to acinus cell necroses and the propagation of the necrotizing inflammation by impaired microcirculation of pancreatic tissue compartments in the initial 48-72 h, are still unknown to a large extent. Hence, management of human AP has been empiric and conflicting opinions are still present regarding medical and surgical management concepts.
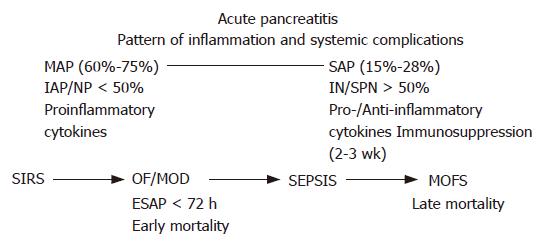
The tissue response of the pancreas to an injury like acinus cell necrosis leads to production and liberation of proinflammatory cytokines, chemokines and other biological active compounds[1-4]. Clinical and experimental studies have verified activation of local macrophages and attraction of activated polymorphonuclear cells (PMNs) as first-line players in the defense and limitation of pancreatic tissue injury[5-7]. Inflammatory mediators are primarily released from the splanchnic area and gain access to the systemic compartment mainly by lymphatic, portal vein and suprahepatic circulation[1,8]. The lungs are the first pass taker of the porto-hepatic blood and lymph of the splanchnic compartments enriched of activated PMNs, cytokines and other biological active compounds. Gut barrier failure, with the ensuing translocation of bacteria and endotoxin, has been proposed as a major contributor to the development of local infection and multi-organ failure in SAP[9]. Evidence of the association between gut injury and the subsequent development of infected necroses and distant organ failure continues to increase. Intestinal permeability disturbances have been found in humans with SAP 72 h after onset, correlating strongly with clinical outcome[10]: the increase of permeability was significantly higher in patients who developed multi-organ failure and/or died compared to patients suffering from mild attacks[11]. Intestinal permeability increases gradually during the course of SAP reaching a maximum at the end of the first week[12]. In a recent prospective study of patients with AP, endotoxemia as a consequence of increase of gut permeability was found on the day of admission to the hospital significantly more common and of greater magnitude in severe attacks than in those with mild attacks, in non-survivors than in survivors, and in patients who developed multi-organ failure than in those who did not[13,14] (Table 1).
| Local | Systemic consequences |
| Mucosal ischemia[10,15,16] | Priming of neutrophils[20-22] |
| Disruption of mucosal epithelial integrity[17] | Endotoxemia[14,23,24] |
| Reperfusion injury of mucosal epithelia[18] | Bacterial translocation[25-27] |
| Increase of intestinal permeability[19] | Cytokine overproduction[1,2,28] |
| Gram-negative intestinal bacterial | Impaired systemic immunity[29,30] |
| overgrowth[11] | |
| Impaired mucosal immunity[11] |
The peritoneal compartment is the site of pro-inflammatory reaction to pancreatic necrosis, whereas an anti-inflammatory response dominates in the lymph collected from the thoracic duct, as well as in the systemic circulation during the first week after onset of systemic complications[1]. The cytokine levels in the blood and lymph are closely associated with the severity of illness on admission, the magnitude of multi-organ failure syndrome (MOFS) and the outcome as well[1,2]. Correlated with local and systemic complications, a compartmentalization of the inflammatory responses has been objectified. Local inflammatory cytokines at high concentrations are found in the portal and splanchnic circulation at the same time when in the systemic blood compartment anti-inflammatory compounds prevail over inflammatory cytokines. In SAP, a compensatory anti-inflammatory response affects the immunocompetence[30,31]. Patients with SAP show impaired immune response in regard to reduced HLA-DR expression of monocytes and macrophages[29,32], reduced numbers of CD4- and CD8-positive T-cells[33,34], an impairment of mononuclear phagocytic capacity[35] and an increase of the anti-inflammatory cytokine IL-10 and IL-1 receptor antagonist[36]. Reduced immune competence, as objectified by reduced expression of HLA-DR of monocytes and macrophages, predicts the development of organ failure and is associated with secondary infection[29] (Figure 1).
At the beginning of the 1980s, the morphologic feature of SAP was established by the definition of infected and sterile necrosis, pancreatic abscess and postacute pseudocysts as the principal morphologic and bacteriologic criteria for clinical severity and considered to be determinants of the clinical course[37]. Macroscopically, necrotizing pancreatitis is characterized by focal or diffuse areas of devitalized parenchyma frequently associated with peripancreatic fatty tissue necroses extending sometimes to retroperitoneal spaces up to the pelvis. Intrapancreatic hemorrhage is variably present and may lead to an acute abdominal compartment syndrome. Infection of necrosis occurs in up to 30% of all patients with necrotizing pancreatitis[27,38]. In the clinical setting of AP, post-acute pseudocysts and pancreatic abscess are late consequences of the disease[39]. In both subgroups of AP, an inflammatory wall is developed which separates the inflammatory processes from the surrounding tissues. Both features have differences in clinical symptomatology and associated morbidity. Peripancreatic fluid collection that arises early during the course of AP is frequently a sign of severity. However, in most instances peripancreatic fluid collection disappears without any treatment[40].
The Atlanta Classification is accepted worldwide as the first clinical reliable classification system of AP. But the accumulation of clinical data forces a revision of the Atlanta criteria of severity. Organ failure has been recognized as a more important determinant of survival than the extent of pancreatic necroses. Particularly early multi-organ failure at admission or in the first days predicts strongly the clinical course and the outcome. Severity of organ failure using multi-step criteria as introduced for septic patients by the SOFA-score is considered clinically relevant and increasingly applied for severity scoring and predicting outcome[41]. The SOFA criteria for systemic organ dysfunctions are clinically more reliable for decision making than the Atlanta criteria.
Acute pancreatitis is not a stable disease. Increasing amounts of intrapancreatic and retroperitoneal necroses are closely related to the frequency and severity of local and systemic complications[27]. About 70%-80% of AP takes a mild course and is associated only with minimal organ dysfunctions. First clinical signs are abdominal pain located in the epigastrium, frequently radiating into the midback (Table 2). Clinical improvement can easily be achieved by fluid replacement, a pain treatment and re-institution of regular food intake. The initial 2-4 d after onset of symptoms are most important, when about 15%-25% of patients with AP take the course of a severe disease. Based on clinical and experimental data, this period is characterized by an initial hypovolaemic state[42-44]. In SAP, hypotension or even shock occurs as a consequence of sequestration of protein-rich fluids into the pancreas, the retroperitoneal spaces and the abdominal cavity. The initial systemic inflammatory response syndrome causes a hyperinflammatory reaction exerting systemic organ dysfunctions of the lungs, kidneys, cardiocirculatory system and splanchnic intestinal compartments[45,46]. Acute fluid collections arise early in the course of severe acute pancreatitis, lack a well-defined wall and are usually peripancreatic in location, and usually resolve without sequelae but may evolve into pancreatic pseudocysts or abscesses. Acute fluid collections rarely require drainage. About 60%-70% of fluid collection resolves spontaneously and has no connection with the pancreatic duct system[47].
| Clinical | Pathophysiologic process | |
| Early: d 1-10 after HA | Hypovolemia Abdominal pain | Fluid sequestration Liberation of pro- and anti- |
| ESAP in about 20% of SAP | Dysfunction Pulmonary Renal | inflammatory cytokines Endotoxemia |
| Cardiocirculatory | Liberation of vasoactive substances | |
| Liver | Disturbance of blood coagulation | |
| Intestine | Translocation of endotoxin and bacteria | |
| Late > 2 wk after HA | Local and systemic | Bacterial translocation |
| septic complications | CARS | |
| IN, SPN | Anti-inflammatory reaction Immunosuppression | |
About 20% of patients with SAP develop in the 72 h after onset of the disease organ failure or even have organ- or multi-organ failure at admission to hospitals[48]. Despite application of maximum intensive care treatment, 30%-50% of the patients with early severe acute pancreatitis do not promptly respond to ICU treatment and take a complicated course with persistence of multi-system organ dysfunctions. Patients suffering from early and persistent multi-organ insufficiency syndrome have a high risk of mortality[49]. Recently it has been shown that severe organ failure within the first week after onset of AP before any kind of intervention is closely linked to clinically relevant pancreatic infection which occurs two weeks later[50]. Early multi-organ dysfunction syndrome (MODS) obviously triggers additional mechanisms that render bacterial translocation into clinically manifested sepsis. Early onset MODS > 2 organs has proved to be the predominant risk factor for death. Early mortality in the first 6-10 d of SAP is caused by severe inflammatory response syndrome (SIRS) associated with early multi-organ insufficiency syndrome (Table 3). Early mortality was reported between 42% and 60%[51-53].
| Admission | Dynamic of organ failure | Hospital mortality | |||
| ESAP (n = 47) | SOF | 25 (53%) | Reversible 9 develop MOF 14 (30%) | 42% | |
| MOF | 22 (47%) | Reversible 1 progress to MOFS 21 (95%) | |||
| SAP (n = 111) | OF (-) | 30 (27%) | |||
| SOF | 26 (23%) | 14% | |||
| MOF | 55 (50%) | ||||
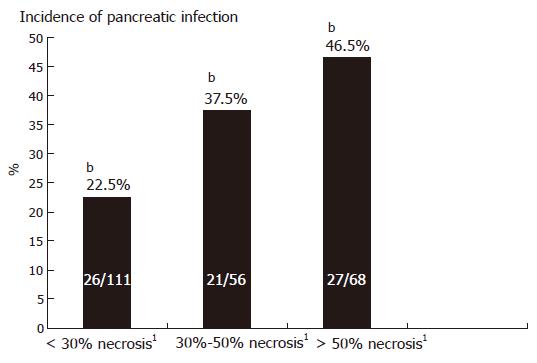
Gross destruction of the pancreatic gland by tissue necroses is observed in about 20% of patients with AP and takes place in the first week after onset[54]. Experimental and clinical observations reveal that development of pancreatic necrosis is accompanied by an increase of local and systemic organ complications, increasing the risk of morbidity and mortality compared to patients with interstitial-oedematous pancreatitis[55,56] (Figure 2). Most patients who develop early or late organ failure suffer from necrotizing pancreatitis. Autopsy data and surgical results have verified that more than 80% of deaths are correlated with the presence of necroses. The highest risk for local and systemic complications is seen in patients who show extended necrosis of more than ≥ 50% of the pancreas by magnetic resonance tomography (MRT) or contrast-enhanced computer tomography (CECT)[57,58]. Patients with sterile extended pancreatic necroses (> 50%) display clinically signs of sepsis including organ dysfunctions, septic fever, leucocytosis, hyperdynamic cardiocirculatory state and intestinal motility disorder. To discriminate clinical infection from sterile necrosis, the use of sepsis criteria is not reliable in patients with extended sterile necrosis.
In addition to the presence of pancreatic parenchymal necrosis, the occurrence and extent of the necrotizing process into extrapancreatic retroperitoneal fatty tissue spaces including tissue compartments of the mesentery of the small and large bowel, the peri-renal fat and the para- and retrocolic compartments, are important factors influencing the course of the disease and strongly affect the clinical severity[59]. The overall infection rate of pancreatic tissue in necrotizing pancreatitis is up to 30% and may increase to 70% in the 3rd wk[27] (Table 4).
| NP (%) | AP (%) | ||
| Infected necrosis | 99 | 23.2 | 6.9 |
| Pancreatic abscess | 40 | 9.4 | 2.8 |
| Infected pseudocyst | |||
| after AP | 7 | 1.6 | 0.5 |
| Total | 146 | 34.2 | 10.1 |
The setting of pancreatic infection includes infected necrosis, pancreatic abscess and infected pancreatic pseudocysts. The bacteriological analysis of puncture aspirates or of intraoperative smears reveals predominantly gram-negative microbes deriving from the intestine. Escherichia coli was the most frequent pathogen followed by Enterococcus and Klebsiella[27]. However, in recent years a shift of the bacterial pattern has been observed towards more gram-positive bacteria like Staph aureus and Enterobacteriaceae[60,61]. The presence of candida species in infected necroses has been observed in 5%-15%[62]. Candida patients have a higher mortality and experience more systemic complications than patients without candida infections of necroses. Recent data about the routine use of prophylactic antibiotics provided evidence that application of antibiotics contributes to the development of candida infection and to changes in bacterial spectrum of infected necroses with an increased incidence of gram-positive infections[62,63].
CT-guided fine needle aspiration (FNA) of the necrotic area is a safe procedure to diagnose infection, identify bacteria and institute appropriate therapy. To distinguish pancreatic inflammation from secondary infection, gram staining and culture must be performed after guided aspiration[64]. The knowledge of the bacteria and candida species and the pattern of chemo-resistance may lead to a rational antibiotic treatment.
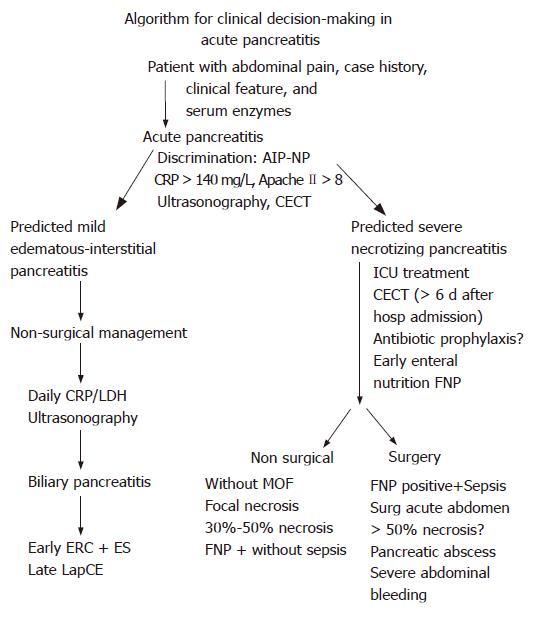
The management of patients with AP is challenging due to late hospitalization after onset of the acute attack and difficulty in distinguishing mild from severe disease in the first 48-72 h. Identification of risk factors for the development of necrotizing pancreatitis within the initial 24 h of hospitalization is of potential clinical importance. Patients who display at admission organ dysfunctions or an Apache II score ≥ 8[65] or C-reactive protein (CRP) > 120 mg/dL[66] or procalcitonin > 1.8 ng/mL[67] or a hematocrit > 44[44] should have early intensive care for optimal surveillance and ICU treatment. The use of early CECT or MRI for determination of severity is limited by several factors: (a) only a quarter of patients with acute pancreatitis develop necroses; (b) pancreatic necroses may not develop in the first 48 h; and (c) the presence of pancreatic necroses and the amount of necroses does not strongly correlate with the development of organ failure (Figure 3)[68]. The CECT based Balthazar classification shows the highest diagnostic and predictive accuracy when performed after the first week of disease. The APACHE II scoring and the sequential Sepsis-Related Organ Failure Assessment (SOFA) have a highly reliable sensitivity and specificity and positive predictive value for the degree of severity of SAP. APACHE II-, Marshall- and SOFA score can objectify the responses of the patients to intensive care measures (Table 5) on a daily basis. The biochemical parameters, CRP, procalcitonin and IL-8 have a high predictive accuracy for the degree of severity of necrotising pancreatitis in the first days. Procalcitonin > 1.4 ng/mL has a diagnostic accuracy of 70% for infection of necrosis; and procalcitonin of > 3.8 ng/mL predicts MODS with a diagnostic accuracy of 92%[71] (Table 6).
| Cut-off | Sensitivity(%) | Specificity(%) | Accuracy(%) | |
| Prediction of infected necrosis | ||||
| PCT | ≥ 1.4 ng/mL | 75 | 68 | 69a |
| CRP | ≥ 400 mg/L | 29 | 92 | 76 |
| Prediction of infected necrosis and MODS | ||||
| PCT | ≥ 3.8 ng/mL | 80 | 93 | 92a |
| CRP | ≥ 410 mg/L | 35 | 93 | 87 |
| Prediction of death | ||||
| PCT | ≥ 3.8 ng/mL | 82 | 88 | 88a |
| Prediction of IN and MODS or death | ||||
| PCT | ≥ 3.8 ng/mL | 76 | 94 | 92a |
| CRP | ≥ 400 mg/L | 35 | 92 | 84 |
Admission hematocrit of > 47 and a failure of admission hematocrit to decrease at 24 h has been identified as reliable criteria of hemoconcentration of SAP in the very early period of the disease[42-44]. Vigorous intravenous fluid resuscitation is required to overcome systemic hypovolemia caused by intravascular fluid loss[72,73]. Intravenous fluid substitution for patients with predicted SAP should be established with 250-350 mL/h for the first 48 h[42]. Restoration of normal cardiocirculatory functions objectified by heart-rate, systolic or mean arterial blood pressure, an oxygen saturation of venous blood of > 95%, absence of a base deficit > 5 μmol/L and urine flow of ≥ 50 mL/h are decisive criteria of treatment response.
In mild biliary acute pancreatitis, endoscopic retrograde cholangiopancreatography (ERCP) and removal of common bile duct stones do not change the natural course of pancreatitis. ERCP, endoscopic sphincterotomy and stone removal are applied after subsidence of clinical signs of AP. In severe biliary pancreatitis, early sphincterotomy and stone extraction are beneficial when common bile duct stone has been diagnosed to be associated with SAP. Early endoscopic extraction of common bile duct stones brings about disappearance of cholestasis and decompression of the pancreatic main duct. A significant reduction of biliary and systemic morbidity has been objectified by two randomized controlled trials (RCTs)[74-76]. However, ERCP and sphincterotomy in SAP increases the risk of an additional pancreatitic trauma in up to 10% of patients and may increase the risk of additional cholangitis episodes during the course of SAP.
Antibiotic prophylaxis turned out to be not very effective in regard to avoidance or reduction of infection of necrosis and associated systemic complications[77]. Two randomized double blinded prospective controlled multi-center trials proved antibiotic prophylaxis ineffective in regard to reduction of infection of necrosis and hospital mortality[78,79] (Table 7). But patients with pulmonary infection and who show a positive blood culture associated with signs of sepsis should be treated with antibiotics. Enteral feeding (EN) in SAP reduces significantly the infection rate of necrosis and lowers the need for surgical interventions[80-84]. However, hospital mortality and non-infectious complications are not altered by enteral feeding compared to parenteral nutrition (Table 8). The beneficial effect of EN may be more pronounced if it is instituted early[85].
| Benefits of enteral nutrition | Lower infections (P = 0.004) |
| Reduced surgical interventions (P = 0.05) | |
| Reduced LHS-2.9 d (P < 0.001) | |
| Differences | Hospital mortality (P = 0.3) |
| Non-infectious complications (P = 0.16) |
Non-surgical ICU-management is successful in most patients with AP who have sterile pancreatic necroses and who do not develop organ failure (Table 9). Patients having pancreatic necroses and who are fine needle procedure (FNP)-positive but do not show clinical signs of sepsis, do not need surgical intervention[86-88].
| Patients | Conservative | Surgery/Intervention | |
| Interstitial-oedematous | 1071 (68.3) | 1056 ( 98.6) | 15 (1.4)2 |
| Necrotizing pancreatitis | 359 (22.9) | 95 (26.5) | 264 (73.5) |
| Sterile necrosis | 227 | 85 ( 37.5) | 142 (62.5) |
| Infected necrosis | 132 | 10 (7.6) | 122 (92.4) |
| Pancreatic abscess | 42 (2.7) | 3 (7.1) | 39 (92.9) |
| Postacute pseudocyst | 96 (6.1) | 22 ( 22.9) | 74 (77.1) |
Surgical debridement has been documented to be effective for patients with proven infected necrosis and progressive clinical sepsis. Patients with SAP who develop a surgical acute abdomen during the course of ICU treatment need emergency surgery to avoid development of abdominal compartment syndrome[57] or consequences of intestinal perforations. Patients with extended sterile necrosis (> 50% of the pancreas) are at high risk for infected necrosis with the consequence of progressive MODS. These patients are candidates for surgical and interventional measures after their clinical signs show non-response to maximum intensive care treatment. Patients with infected necrosis are managed by surgical and interventional treatment modalities (Table 10).
| Complication | Hospital mortality | |||
| n | Postop, n (%) | Reop, n (%) | n (%) | |
| Pederzoli 1990[90] | 191 | 55 (29) | 34 (18 ) | 40 (21) |
| Beger 1999[92] | 221 | 122 (55) | 93 (42) | 46 (21) |
| Mai 2000[61] | 27 | 10 (37) | 6 (22) | 5 (18) |
| Hungness 2002[93] | 26 | 4 (15.4) | 6 (23) | |
| Farkas 2006[94] | 220 | 43% | 48 (22) | 17 (7.7) |
| Howard 2007[95] | 102 | 83 (81) | 69 (68) | 12 (11.8) |
| 1990-2007 | 787 | 43% | 29.60% | 14.70% |
A variety of surgical treatment modalities are currently in use. The advantages of minimally invasive interventional debridement, whether performed by laparoscopic techniques or by a retroperitoneal approach, are up to now not based on results of controlled clinical trials. By use of minimal invasive techniques for infected necrosis in the late course of disease, the morbidity remains high for several days. Two to 7 reoperations with lavage are necessary to interrupt systemic complications of the local inflammatory process[95-102] (Table 11). An open surgical debridement combined with continuous short-term lavage of the lesser sack interrupts clinical sepsis in patients suffering from extended necrosis with infection accompanied by multi-system organ failure. The early treatment related morbidity is much lower in patients treated with open surgery than after first pass of minimal invasive debridement. The frequency of reoperation is between 25% and 40% and up to 100% in patients after minimal access intervention. Hospital mortality in high volume centers is below 20% after open necrosectomy plus bursa lavage and after minimal invasive surgical approach as well.
| n | Infect.necrosis(%) | ApacheII | Time0-S | Earlymorbidity(%) | OP/pts | Hospitalmortality(%) | |
| Freeny 1998[96] | 34 | 100 | - | 20 | |||
| Goiuzi 1999[97] | 32 | 81 | 26 | 15 | |||
| Carter 2000[98] | 10 | 90 | 24 d | 10 | 3 | 20 | |
| Horvath 2001[99] | 6 | 100 | 33 | 0 | |||
| Castellanos 2002[100] | 15 | 100 | 40 | 27 | |||
| Connor 2003[101] | 24 | 58 | 8 | 88 | 4 | 25 | |
| Zhou 2003[102] | 12 | 58 | 72/102 | 0 | |||
| Connor 2005[102] | 47 | 81 | 9 | 28 d | 92 | 3 | 19 |
| 1998-2006 | 156 pts | 83 | 70.6 | 18.3 |
S- Editor Zhu LH L- Editor Zhu LH E- Editor Wang HF
| 1. | Dugernier TL, Laterre PF, Wittebole X, Roeseler J, Latinne D, Reynaert MS, Pugin J. Compartmentalization of the inflammatory response during acute pancreatitis: correlation with local and systemic complications. Am J Respir Crit Care Med. 2003;168:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Mayer J, Rau B, Gansauge F, Beger HG. Inflammatory mediators in human acute pancreatitis: clinical and pathophysiological implications. Gut. 2000;47:546-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 307] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 3. | Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 257] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 4. | Lipsett PA. Serum cytokines, proteins, and receptors in acute pancreatitis: mediators, markers, or more of the same? Crit Care Med. 2001;29:1642-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Pezzilli R, Maldini M, Morselli-Labate AM, Barakat B, Romboli E, Beltrandi E, Migliori M, Tomassetti P, Corinaldesi R. Early activation of peripheral lymphocytes in human acute pancreatitis. J Clin Gastroenterol. 2003;36:360-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Poch B, Gansauge F, Rau B, Wittel U, Gansauge S, Nüssler AK, Schoenberg M, Beger HG. The role of polymorphonuclear leukocytes and oxygen-derived free radicals in experimental acute pancreatitis: mediators of local destruction and activators of inflammation. FEBS Lett. 1999;461:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Sakai Y, Masamune A, Satoh A, Nishihira J, Yamagiwa T, Shimosegawa T. Macrophage migration inhibitory factor is a critical mediator of severe acute pancreatitis. Gastroenterology. 2003;124:725-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Guzman EA, Rudnicki M. Intricacies of host response in acute pancreatitis. J Am Coll Surg. 2006;202:509-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Deitch EA, Xu DZ, Qi L, Berg RD. Bacterial translocation from the gut impairs systemic immunity. Surgery. 1991;109:269-276. [PubMed] |
| 10. | Bonham MJ, Abu-Zidan FM, Simovic MO, Windsor JA. Gastric intramucosal pH predicts death in severe acute pancreatitis. Br J Surg. 1997;84:1670-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Ammori BJ. Role of the gut in the course of severe acute pancreatitis. Pancreas. 2003;26:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 12. | Juvonen PO, Tenhunen JJ, Heino AA, Merasto M, Paajanen HE, Alhava EM, Takala JA. Splanchnic tissue perfusion in acute experimental pancreatitis. Scand J Gastroenterol. 1999;34:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Holland J, Carey M, Hughes N, Sweeney K, Byrne PJ, Healy M, Ravi N, Reynolds JV. Intraoperative splanchnic hypoperfusion, increased intestinal permeability, down-regulation of monocyte class II major histocompatibility complex expression, exaggerated acute phase response, and sepsis. Am J Surg. 2005;190:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Ammori BJ, Leeder PC, King RF, Barclay GR, Martin IG, Larvin M, McMahon MJ. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg. 1999;3:252-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 235] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Wang XD, Wang Q, Andersson R, Ihse I. Alterations in intestinal function in acute pancreatitis in an experimental model. Br J Surg. 1996;83:1537-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Soong CV, Halliday MI, Barclay GR, Hood JM, Rowlands BJ, Barros D'Sa AA. Intramucosal acidosis and systemic host responses in abdominal aortic aneurysm surgery. Crit Care Med. 1997;25:1472-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Haglund U, Lundgren O. Intestinal ischemia and shock factors. Fed Proc. 1978;37:2729-2733. [PubMed] |
| 18. | Horton JW, Walker PB. Oxygen radicals, lipid peroxidation, and permeability changes after intestinal ischemia and reperfusion. J Appl Physiol (1985). 1993;74:1515-1520. [PubMed] |
| 19. | Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg. 1996;20:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 424] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 20. | Norman J, Franz M, Messina J, Riker A, Fabri PJ, Rosemurgy AS, Gower WR. Interleukin-1 receptor antagonist decreases severity of experimental acute pancreatitis. Surgery. 1995;117:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | de Beaux AC, Goldie AS, Ross JA, Carter DC, Fearon KC. Serum concentrations of inflammatory mediators related to organ failure in patients with acute pancreatitis. Br J Surg. 1996;83:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 181] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Guice KS, Oldham KT, Caty MG, Johnson KJ, Ward PA. Neutrophil-dependent, oxygen-radical mediated lung injury associated with acute pancreatitis. Ann Surg. 1989;210:740-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Exley AR, Leese T, Holliday MP, Swann RA, Cohen J. Endotoxaemia and serum tumour necrosis factor as prognostic markers in severe acute pancreatitis. Gut. 1992;33:1126-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 162] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Windsor JA, Fearon KC, Ross JA, Barclay GR, Smyth E, Poxton I, Garden OJ, Carter DC. Role of serum endotoxin and antiendotoxin core antibody levels in predicting the development of multiple organ failure in acute pancreatitis. Br J Surg. 1993;80:1042-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Steffen EK, Berg RD. Relationship between cecal population levels of indigenous bacteria and translocation to the mesenteric lymph nodes. Infect Immun. 1983;39:1252-1259. [PubMed] |
| 26. | Koh IH, Montero EF, Keller R, Silva MH, Goldenberg S, Silva RM. Can bacterial translocation to the mesenteric lymph node be correlated with systemic infection? Transplant Proc. 1996;28:2673. [PubMed] |
| 27. | Beger HG, Bittner R, Block S, Büchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology. 1986;91:433-438. [PubMed] |
| 28. | Banks RE, Evans SW, Alexander D, Van Leuven F, Whicher JT, McMahon MJ. Alpha 2 macroglobulin state in acute pancreatitis. Raised values of alpha 2 macroglobulin-protease complexes in severe and mild attacks. Gut. 1991;32:430-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Mentula P, Kylänpää ML, Kemppainen E, Jansson SE, Sarna S, Puolakkainen P, Haapiainen R, Repo H. Plasma anti-inflammatory cytokines and monocyte human leucocyte antigen-DR expression in patients with acute pancreatitis. Scand J Gastroenterol. 2004;39:178-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Kylänpää-Bäck ML, Takala A, Kemppainen E, Puolakkainen P, Kautiainen H, Jansson SE, Haapiainen R, Repo H. Cellular markers of systemic inflammation and immune suppression in patients with organ failure due to severe acute pancreatitis. Scand J Gastroenterol. 2001;36:1100-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Bhatnagar A, Wig JD, Majumdar S. Immunological findings in acute and chronic pancreatitis. ANZ J Surg. 2003;73:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Mentula P, Kylänpää-Bäck ML, Kemppainen E, Takala A, Jansson SE, Kautiainen H, Puolakkainen P, Haapiainen R, Repo H. Decreased HLA (human leucocyte antigen)-DR expression on peripheral blood monocytes predicts the development of organ failure in patients with acute pancreatitis. Clin Sci (Lond). 2003;105:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Widdison AL, Cunningham S. Immune function early in acute pancreatitis. Br J Surg. 1996;83:633-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Sweeney KJ, Kell MR, Coates C, Murphy T, Reynolds JV. Serum antigen(s) drive the proinflammatory T cell response in acute pancreatitis. Br J Surg. 2003;90:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Larvin M, Alexander DJ, Switala SF, McMahon MJ. Impaired mononuclear phagocyte function in patients with severe acute pancreatitis: evidence from studies of plasma clearance of trypsin and monocyte phagocytosis. Dig Dis Sci. 1993;38:18-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Granger J, Remick D. Acute pancreatitis: models, markers, and mediators. Shock. 2005;24 Suppl 1:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 180] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 37. | Bradley EL. A clinically based classification system for acute pancreatitis. Ann Chir. 1993;47:537-541. [PubMed] |
| 38. | Fedorak IJ, Ko TC, Djuricin G, McMahon M, Thompson K, Prinz RA. Secondary pancreatic infections: are they distinct clinical entities? Surgery. 1992;112:824-830; discussion 830-831. [PubMed] |
| 39. | Bittner R, Block S, Büchler M, Beger HG. Pancreatic abscess and infected pancreatic necrosis. Different local septic complications in acute pancreatitis. Dig Dis Sci. 1987;32:1082-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 121] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med. 1994;330:1198-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 597] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 41. | Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6591] [Cited by in RCA: 7756] [Article Influence: 267.4] [Reference Citation Analysis (1)] |
| 42. | Eckerwall G, Olin H, Andersson B, Andersson R. Fluid resuscitation and nutritional support during severe acute pancreatitis in the past: what have we learned and how can we do better? Clin Nutr. 2006;25:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Baillargeon JD, Orav J, Ramagopal V, Tenner SM, Banks PA. Hemoconcentration as an early risk factor for necrotizing pancreatitis. Am J Gastroenterol. 1998;93:2130-2134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 110] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Brown A, Baillargeon JD, Hughes MD, Banks PA. Can fluid resuscitation prevent pancreatic necrosis in severe acute pancreatitis? Pancreatology. 2002;2:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Marshall JC, Christou NV, Meakins JL. The gastrointestinal tract. The "undrained abscess" of multiple organ failure. Ann Surg. 1993;218:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 297] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 46. | Goris RJ, te Boekhorst TP, Nuytinck JK, Gimbrère JS. Multiple-organ failure. Generalized autodestructive inflammation? Arch Surg. 1985;120:1109-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 629] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 47. | Traverso LW, Kozarek RA. Interventional management of peripancreatic fluid collections. Surg Clin North Am. 1999;79:745-757, viii-ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Isenmann R, Rau B, Beger HG. Early severe acute pancreatitis: characteristics of a new subgroup. Pancreas. 2001;22:274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 130] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 49. | Tao HQ, Zhang JX, Zou SC. Clinical characteristics and management of patients with early acute severe pancreatitis: experience from a medical center in China. World J Gastroenterol. 2004;10:919-921. [PubMed] |
| 50. | Rau BM, Bothe A, Kron M, Beger HG. Role of early multisystem organ failure as major risk factor for pancreatic infections and death in severe acute pancreatitis. Clin Gastroenterol Hepatol. 2006;4:1053-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 51. | McKay CJ, Evans S, Sinclair M, Carter CR, Imrie CW. High early mortality rate from acute pancreatitis in Scotland, 1984-1995. Br J Surg. 1999;86:1302-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 194] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 52. | Renner IG, Savage WT, Pantoja JL, Renner VJ. Death due to acute pancreatitis. A retrospective analysis of 405 autopsy cases. Dig Dis Sci. 1985;30:1005-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 303] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 53. | Wilson C, Imrie CW, Carter DC. Fatal acute pancreatitis. Gut. 1988;29:782-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Block S, Maier W, Bittner R, Büchler M, Malfertheiner P, Beger HG. Identification of pancreas necrosis in severe acute pancreatitis: imaging procedures versus clinical staging. Gut. 1986;27:1035-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 139] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Beger HG, Büchler MW. Decision-making in surgical treatment of acute pancreatitis: operative or consecutive management of necrotizin pancreatitis. Theor Surg. 1986;1:61. |
| 56. | Beger HG, Krautzberger W, Bittner R, Block S. Results of surgical treatment of necrotizing pancreatitis. World J Surg. 1985;9:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 57. | Rau B, Pralle U, Uhl W, Schoenberg MH, Beger HG. Management of sterile necrosis in instances of severe acute pancreatitis. J Am Coll Surg. 1995;181:279-288. [PubMed] |
| 58. | Karimgani I, Porter KA, Langevin RE, Banks PA. Prognostic factors in sterile pancreatic necrosis. Gastroenterology. 1992;103:1636-1640. [PubMed] |
| 59. | Takeda K, Matsuno S, Sunamura M, Kobari M. Surgical aspects and management of acute necrotizing pancreatitis: recent results of a cooperative national survey in Japan. Pancreas. 1998;16:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Rau B, Bothe A, Beger HG. Surgical treatment of necrotizing pancreatitis by necrosectomy and closed lavage: changing patient characteristics and outcome in a 19-year, single-center series. Surgery. 2005;138:28-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 61. | Mai G, Uhl W, Muller CH, Büchler MW. The conservative management of severe acute pancreatitis. Acute Pancreatitis Novel Concepts in Biology and Therapy. Oxford: Blackwell Science Ltd 1999; 475-485. |
| 62. | Isenmann R, Schwarz M, Rau B, Trautmann M, Schober W, Beger HG. Characteristics of infection with Candida species in patients with necrotizing pancreatitis. World J Surg. 2002;26:372-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | He YM, Lv XS, Ai ZL, Liu ZS, Qian Q, Sun Q, Chen JW, Lei DX, Jiang CQ, Yuan YF. Prevention and therapy of fungal infection in severe acute pancreatitis: A prospective clinical study. World J Gastroenterol. 2003;9:2619-2621. [PubMed] |
| 64. | Rau B, Pralle U, Mayer JM, Beger HG. Role of ultrasonographically guided fine-needle aspiration cytology in the diagnosis of infected pancreatic necrosis. Br J Surg. 1998;85:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 114] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 65. | Larvin M, McMahon MJ. APACHE-II score for assessment and monitoring of acute pancreatitis. Lancet. 1989;2:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 426] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 66. | Uhl W, Büchler M, Malfertheiner P, Martini M, Beger HG. PMN-elastase in comparison with CRP, antiproteases, and LDH as indicators of necrosis in human acute pancreatitis. Pancreas. 1991;6:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 118] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 67. | Rau B, Steinbach G, Gansauge F, Mayer JM, Grünert A, Beger HG. The potential role of procalcitonin and interleukin 8 in the prediction of infected necrosis in acute pancreatitis. Gut. 1997;41:832-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 232] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 68. | Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 959] [Article Influence: 27.4] [Reference Citation Analysis (1)] |
| 69. | Rau B, Schilling MK, Beger HG. Laboratory markers of severe acute pancreatitis. Dig Dis. 2004;22:247-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Localio SA. Objective early identification of severe acute pancreatitis. Am J Gastroenterol. 1974;61:443-451. [PubMed] |
| 71. | Rau BM, Kemppainen EA, Gumbs AA, Büchler MW, Wegscheider K, Bassi C, Puolakkainen PA, Beger HG. Early assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT): a prospective international multicenter study. Ann Surg. 2007;245:745-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 72. | Tenner S. Initial management of acute pancreatitis: critical issues during the first 72 hours. Am J Gastroenterol. 2004;99:2489-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 73. | Ruokonen E, Uusaro A, Alhava E, Takala J. The effect of dobutamine infusion on splanchnic blood flow and oxygen transport in patients with acute pancreatitis. Intensive Care Med. 1997;23:732-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | Neoptolemos JP, Carr-Locke DL, London NJ, Bailey IA, James D, Fossard DP. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet. 1988;2:979-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 470] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 75. | Fan ST, Lai EC, Mok FP, Lo CM, Zheng SS, Wong J. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med. 1993;328:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 439] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 76. | Fölsch UR, Nitsche R, Lüdtke R, Hilgers RA, Creutzfeldt W. Early ERCP and papillotomy compared with conservative treatment for acute biliary pancreatitis. The German Study Group on Acute Biliary Pancreatitis. N Engl J Med. 1997;336:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 330] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 77. | de Vries AC, Besselink MG, Buskens E, Ridwan BU, Schipper M, van Erpecum KJ, Gooszen HG. Randomized controlled trials of antibiotic prophylaxis in severe acute pancreatitis: relationship between methodological quality and outcome. Pancreatology. 2007;7:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 78. | Isenmann R, Rünzi M, Kron M, Kahl S, Kraus D, Jung N, Maier L, Malfertheiner P, Goebell H, Beger HG. Prophylactic antibiotic treatment in patients with predicted severe acute pancreatitis: a placebo-controlled, double-blind trial. Gastroenterology. 2004;126:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 298] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 79. | Dellinger P. Antibiotic prophylaxis in severe acute pancreatitis. Annals of Surgery. 2007;In press. |
| 80. | Abou-Assi S, Craig K, O'Keefe SJ. Hypocaloric jejunal feeding is better than total parenteral nutrition in acute pancreatitis: results of a randomized comparative study. Am J Gastroenterol. 2002;97:2255-2262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 177] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 81. | Oláh A, Pardavi G, Belágyi T, Nagy A, Issekutz A, Mohamed GE. Early nasojejunal feeding in acute pancreatitis is associated with a lower complication rate. Nutrition. 2002;18:259-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 82. | McClave SA, Greene LM, Snider HL, Makk LJ, Cheadle WG, Owens NA, Dukes LG, Goldsmith LJ. Comparison of the safety of early enteral vs parenteral nutrition in mild acute pancreatitis. JPEN J Parenter Enteral Nutr. 1997;21:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 254] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 83. | Kalfarentzos F, Kehagias J, Mead N, Kokkinis K, Gogos CA. Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: results of a randomized prospective trial. Br J Surg. 1997;84:1665-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 293] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 84. | Gupta R, Patel K, Calder PC, Yaqoob P, Primrose JN, Johnson CD. A randomised clinical trial to assess the effect of total enteral and total parenteral nutritional support on metabolic, inflammatory and oxidative markers in patients with predicted severe acute pancreatitis (APACHE II & gt; or =6). Pancreatology. 2003;3:406-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 85. | Windsor AC, Kanwar S, Li AG, Barnes E, Guthrie JA, Spark JI, Welsh F, Guillou PJ, Reynolds JV. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998;42:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 395] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 86. | Adler DG, Chari ST, Dahl TJ, Farnell MB, Pearson RK. Conservative management of infected necrosis complicating severe acute pancreatitis. Am J Gastroenterol. 2003;98:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 87. | Manes G, Uomo I, Menchise A, Rabitti PG, Ferrara EC, Uomo G. Timing of antibiotic prophylaxis in acute pancreatitis: a controlled randomized study with meropenem. Am J Gastroenterol. 2006;101:1348-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 88. | Runzi M, Niebel W, Goebell H, Gerken G, Layer P. Severe acute pancreatitis: nonsurgical treatment of infected necroses. Pancreas. 2005;30:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 89. | Pederzoli P, Bassi C, Vesentini S, Girelli R, Cavallini G, Falconi M, Nifosi F, Riela A, Dagradi A. Retroperitoneal and peritoneal drainage and lavage in the treatment of severe necrotizing pancreatitis. Surg Gynecol Obstet. 1990;170:197-203. [PubMed] |
| 90. | Farkas G, Márton J, Mándi Y, Szederkényi E. Surgical strategy and management of infected pancreatic necrosis. Br J Surg. 1996;83:930-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 55] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 91. | Beger HG. Surgical management of necrotizing pancreatitis. Surg Clin North Am. 1989;69:529-549. [PubMed] |
| 92. | Hungness ES, Robb BW, Seeskin C, Hasselgren PO, Luchette FA. Early debridement for necrotizing pancreatitis: is it worthwhile? J Am Coll Surg. 2002;194:740-744; discussion 740-744;. [PubMed] |
| 93. | Farkas G, Márton J, Mándi Y, Leindler L. Surgical management and complex treatment of infected pancreatic necrosis: 18-year experience at a single center. J Gastrointest Surg. 2006;10:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 94. | Howard TJ, Patel JB, Zyromski N, Sandrasegaran K, Yu J, Nakeeb A, Pitt HA, Lillemoe KD. Declining morbidity and mortality rates in the surgical management of pancreatic necrosis. J Gastrointest Surg. 2007;11:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 95. | Freeny PC, Hauptmann E, Althaus SJ, Traverso LW, Sinanan M. Percutaneous CT-guided catheter drainage of infected acute necrotizing pancreatitis: techniques and results. AJR Am J Roentgenol. 1998;170:969-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 302] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 96. | Gouzi JL, Bloom E, Julio C, Labbé F, Sans N, el Rassi Z, Carrère N, Pradère B. Percutaneous drainage of infected pancreatic necrosis: an alternative to surgery. Chirurgie. 1999;124:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 97. | Carter CR, McKay CJ, Imrie CW. Percutaneous necrosectomy and sinus tract endoscopy in the management of infected pancreatic necrosis: an initial experience. Ann Surg. 2000;232:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 268] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 98. | Horvath KD, Kao LS, Wherry KL, Pellegrini CA, Sinanan MN. A technique for laparoscopic-assisted percutaneous drainage of infected pancreatic necrosis and pancreatic abscess. Surg Endosc. 2001;15:1221-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 99. | Castellanos G, Piñero A, Serrano A, Llamas C, Fuster M, Fernandez JA, Parrilla P. Translumbar retroperitoneal endoscopy: an alternative in the follow-up and management of drained infected pancreatic necrosis. Arch Surg. 2005;140:952-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 100. | Connor S, Ghaneh P, Raraty M, Rosso E, Hartley MN, Garvey C, Hughes M, McWilliams R, Evans J, Rowlands P. Increasing age and APACHE II scores are the main determinants of outcome from pancreatic necrosectomy. Br J Surg. 2003;90:1542-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 101. | Zhou ZG, Zheng YC, Shu Y, Hu WM, Tian BL, Li QS, Zhang ZD. Laparoscopic management of severe acute pancreatitis. Pancreas. 2003;27:e46-e50. [PubMed] |
| 102. | Connor S, Raraty MG, Howes N, Evans J, Ghaneh P, Sutton R, Neoptolemos JP. Surgery in the treatment of acute pancreatitis--minimal access pancreatic necrosectomy. Scand J Surg. 2005;94:135-142. [PubMed] |