INTRODUCTION
It has been well established that chronic ethanol consump-tion can lead to a variety of pathological consequences (ethanol-related liver injuries such as hepatomegaly, fatty liver, alcoholic hepatitis and cirrhosis)[1,2], yet the mechanism(s) by which ethanol causes hepatotoxicity requires further clarification. From several years of investigation, certain factors have been identified as having a role in ethanol-induced toxicity, such as changes in redox status (NAD+/NADH ratio), the accumulation of acetaldehyde (a product of ethanol oxidation), depletion of antioxidants such as glutathione, and the generation of reactive oxygen species (ROS)[3-8]. As a consequence, it has been demonstrated that amongst the various deleterious effects that can result from ethanol administration, it has been shown that alcohol-induced diseases are accompanied by morphological liver changes that include the increased production of apoptotic cells[9-11]. More specifically, it has been demonstrated that ethanol administration is linked to hepatocyte apoptosis[12-14] and that the number of apoptotic cells detected in the liver correlates with the development of ethanol-induced pathological liver injury[15].
Apoptosis is a regulated mode of cell death, which is characterized by specific biochemical and morphological changes in the cell. Morphologically, during induction of apoptosis, the affected cells shrink, lose cytoskeletal contacts and undergo chromatin condensation. During the terminal phase of the apoptotic process, the nucleus collapses followed by fragmentation of the entire cell into apoptotic bodies which are recognized and eliminated through phagocytosis by neighboring cells[16-18]. The search for the biochemical and signal transduction pathways involved in producing the characteristic apoptotic morphological cellular changes has been underway for several years. The current dogma is that apoptosis is thought to occur by two main pathways involving either an extrinsic route (which utilizes death receptors), and/or an intrinsic pathway that involves mitochondrial intracellular stress signals[19]. Once triggered, the apoptotic machinery is set into motion through the activation of a family of intracellular cysteine proteases known as caspases, which amplify appropriate signals by acting as initiators as well as executioners in the death program. For instance, there are downstream executioner enzymes (caspases 3, 6, and 7) that are activated by upstream initiator caspases (caspases 8, 2, 9, and 10) that are in turn regulated by specific protein-protein interactions. As an example, caspases may be activated in an extrinsic manner by membrane signaling events (death domain transmembrane receptors) and/or by intracellular intrinsic changes resulting in the release of specific proteins from the mitochondria. Intertwined in these pathways are the proteins that control the intrinsic or extrinsic routes of apoptosis that often belong to families that have specific domains that mediate their action. Some of the regulatory proteins that have been identified include those of the Bcl-2 family, which possess anti-apoptotic as well as pro-apoptotic activity via the Bcl-2 and Bax genes respectively.
Normally, in a healthy individual, the highly regulated apoptotic system is counterbalanced by cytoprotective signals that maintain tissue homeostasis. However, when an organism is subjected to repeated or exacerbated insults of a pathological stimulus such as alcohol, pro-apoptotic death factors can be inappropriately expressed shifting the balance towards enhancement of the apoptotic machinery and subsequent deleterious effects. Hence, it has become clinically relevant that a better understanding of the mechanisms and factors involved in apoptosis be ascertained which has lead to an emergence of studies concerning the role of apoptosis in the initiation and progression of alcohol-induced liver injury.
ETHANOL-MEDIATED PATHOLOGICAL FACTORS AND APOPTOSIS
Over the past decade, significant progress has been made concerning the identification of contributing factors that are involved in the initiation and progression of apoptosis in both clinical and experimental alcoholic liver disease states. In general, it has been shown that alcohol-mediated apoptosis is a multi-factorial process that could involve: (1) oxidative stress mechanisms[20]; (2) the effects of various cytokines, particularly TNF-α and TGF-β[21,22]; (3) the involvement of death receptor pathways (TNF-receptor 1 and Fas/CD95)[7,23]. How and when these factors elicit hepatotoxic effects is not completely understood, however it has been noted that a direct correlation exists between the ethanol concentration and time of exposure in the ability of these factors to promote apoptotic and/or necrotic cell death. For instance, it has been shown that higher amounts of alcohol resulted in a decline in apoptosis with an increase in promotion of necrotic cell death, presumably from the induction of microsomal cytochrome components, specifically cytochrome P450 2E1 (CYP2E1). Conversely, at lower concentrations of ethanol, apoptosis is the preferential mode of injury as death was found to be triggered by the Fas receptor system[24]. Thus, ethanol administration is related to cellular injury mechanisms, yet a complete understanding of the presumably intertwined factors that can be involved in hepatotoxicity is still sought. The following is a brief review of current knowledge concerning the factors identified in alcohol-mediated apoptotic liver damage.
Oxidative stress and alcohol-induced liver apoptosis
Following the recognition that ethanol and its metabolites induce the formation of reactive oxygen species (ROS) in liver cells; studies were able to link the ethanol-mediated induction of oxidative stress to the observed enhancement of apoptosis. Specifically, it has been demonstrated that one of the mechanisms responsible for ethanol-induced hepatotoxicity appears to involve the induction of intracellular enzymes, such as alcohol dehydrogenase (ADH) and the induction of CYP2E1, that oxidize ethanol to reactive metabolites, producing reactive oxygen species and lipid peroxides[20]. In linking the ethanol-induced oxidative stress with liver cell apoptosis, it was determined that ROS cause damage to the mitochondria by altering mitochondrial membrane potential and/or membrane permeability. This in turn can initiate the release of pro-apoptotic factors, such as cytochrome c, thus activating the caspase cascade[25-27]. Identified targets in this process include mitochondrial DNA and specific proteins that are promoters of apoptosis (Bax, Bad, and Bid), which are related to the Bcl-2 oncogene family[7].
Ethanol treatment was also shown to impair antioxidant levels in hepatocytes, resulting in ROS generation and increased oxidative stress. For example, the balance of glutathione is impaired by ethanol treatment and was found to be involved in the regulation of mitochondrial function in alcohol-associated apoptosis[28]. In particular, it was determined that the mitochondrial glutathione (mGSH) pool itself is depleted after ethanol administration, leading to the induction of the apoptotic cell death program[29-31].
Overall, in reviewing the data, it is clear that ethanol administration results in enhanced oxidative stress; that mitochondria are intimately involved in the survival of the cell as both a producer and target of ROS; and that apoptotic death can be the consequential end of these changes.
The role of cytokines in ethanol-induced apoptosis
Cytokines are produced by multiple cell types in the liver and are integral in cellular signaling processes. Normally, liver cells use well-developed defense mechanisms as protection against cytokine-mediated damage. However, when toxins such as ethanol stress the cells, they lose their survival protection and become susceptible to the effects of the various cytokines[21].
This scenario has been shown as the ethanol-related release of cytokines [e.g. tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), IL-6, IL-8, IL-10, and transforming growth factor-beta 1, TGF-β1] is associated with the promotion of pro-apoptotic mechanisms[22,32]. Furthermore, TNF-α and TGF-β in particular have been strongly implicated in ethanol-induced apoptosis. Specifically, it has been well documented that the chronic exposure of ethanol can result in an elevation of hepatic TNF-α, which in turn has been related to the activation of caspases and the apoptotic program[8,33,34]. One pathway in which TNF-α-associated apoptosis occurs is through the interaction with the death receptor family, Fas ligand/Fas, and TNF/TNF receptor (TNFR) systems. Within this family are three characterized receptors (TNFR1, TNFR2, and the Fas antigen, which are structurally related with shared homology for a death domain sequence, a C-terminal intracellular domain that is involved in apoptosis pathways. In another mechanism of TNF-α-induced cell death, the role of TNF-α and mitochondrial GSH depletion in ethanol-related apoptosis was further defined. As mentioned above, ethanol impairs the transport of cellular glutathione (particularly mGSH) resulting in its depletion and the subsequent sensitization of the cell to undergo an apoptotic death. This ethanol-related depletion of mGSH was also found to be associated with TNF-mediated cell death in hepatocytes, as the cells became apoptotic as a result of TNF-related events (such as the generation of hydrogen peroxide). Validation of this association came through studies demonstrating that TNF-α-induced oxidative stress and subsequent apoptosis could be prevented by incubating hepatocytes with antioxidants as well as S-adenosyl-L-methionine, a substance that increases mGSH levels[35]. Overall, the data obtained from numerous studies has demonstrated that ethanol exacerbates TNF-related hepatotoxicity, in part, through the induction and perpetuation of apoptotic cell death pathways.
Participation of TGF-β1 in ethanol-induced apoptosis has also been widely studied. TGF-β1 is the prototype member of the TGF-β family that is involved in a diverse array of biological activities including development, differentiation, tissue remodeling, and apoptosis[36,37]. Several liver cells (including hepatocytes and hepatic stellate cells) can produce TGF-β1 in response to alcohol toxicity, and this production is considered to have a significant impact in the initiation and progression of alcoholic liver disease[38,39]. Researchers have identified some of the pro-apoptotic pathways that have been induced by the up-regulation of TGF-β1 in response to ethanol treatment. It has been demonstrated utilizing a fetal hepatocyte model that TGF-β1 induces apoptosis by producing oxidative stress in the cells by increasing ROS production and decreasing the level of a natural antioxidant, glutathione[40,41]. In addition, the increase in TGF-β1-mediated signaling, resulting in caspase activation, can be associated with the enhanced cleavage of the caspase substrate PARP [poly (ADP)-ribose polymerase][42]. It has also been identified that TGF-β1 can activate the caspase cascade by either of the two primary pro-apoptotic mechanisms (death receptor-mediated pathway or intracellular stress-signaled pathway involving mitochondrial changes and the release of cytochrome c), and either pathway can involve the formation of an apoptosome complex that activates the proteolysis of the cell[43].
Involvement of the Fas/Fas ligand system in ethanol-induced liver cell apoptosis
Of the death receptor-mediated pathways, the involvement of the Fas/Fas ligand system has proven to play a significant role in alcohol-related apoptosis in the liver. The pro-apoptotic signaling of Fas (a glycosylated cell-surface protein) is similar to other death receptors in that a specific interaction of the oligomerized receptor with an associated ligand (i.e. Fas ligand) or antibody stimulates the recruitment of the cytoplasmic adapter protein (Fas associated death domain, FADD) that mediates caspase activation and the signaling of the apoptotic death program[44]. In the liver, increases in the expression of membrane-bound Fas as well as the levels of soluble Fas and Fas ligand have been associated with pathological conditions associated with alcoholic hepatitis[13,45]. Also, the up-regulation of Fas ligand in hepatocytes following ethanol treatment is thought to induce apoptotic death of neighboring cells by interacting with the Fas receptor on the surface of those cells[45]. Overall, it is thought that the activation of Fas results in the promotion of the apoptotic program that can include the induction of the caspase cascade and permeabilization of the mitochondrial membrane[46,11]. Moreover, the importance of the Fas/Fas ligand system in caspase 3 activation and apoptosis in the liver following ethanol treatment was substantiated as the administration of a caspase 3 inhibitor was shown to block ethanol-induced caspase 3 activity along with apoptosis[47]. Thus, there is a growing amount of evidence that the Fas/Fas ligand system is a critical element in the activation of the caspase cascade and the subsequent demise of liver cells following chronic ethanol abuse.
MECHANISMS OF ALCOHOL-INDUCED APOPTOSIS
The enhancement of our understanding of the mechanisms involved in the apoptotic cascade is becoming exceedingly important and relevant to disease states such as ALD. However, studies concerning the effect of ethanol on cellular processes such as apoptosis are often limited due to the applicability of the model system. As an example, the dose required for ethanol-induced hepatotoxicity in vitro has been found to vary between cell strains presumably due to the differences in the metabolism of ethanol by ADH and CYP2E1[25]. Also, the use of freshly isolated hepatocyte models were found to produce confounding results when searching for mechanistic parameters as the cells dedifferentiate (i.e. lose cell polarity and many liver-specific characteristics such as the ability to metabolize ethanol) within a few hours after isolation, making the cells useful for short-term culture conditions only. Despite these limitations, many studies have been performed yielding useful information concerning pro-apoptotic mechanisms that are induced following ethanol administration.
Around fifteen years ago, interest began to develop concerning the relevance of apoptotic cell death in liver pathology with particular interest in hepatocellular injury associated with ethanol toxicity[48]. Since the acceptance that apoptosis may play a significant role in the development of liver injury, studies have searched for the potential mechanisms and pathways that are involved. In animal models, it was demonstrated that ethanol feeding resulted in an increase in apoptotic cells in the liver[11,49] that was subsequently shown to be related to the induction of reactive oxygen species (ROS) as a result of ethanol metabolism and the generation of acetaldehyde[50]. Furthermore, it was concluded that the ethanol-induced ROS production was driven by redox changes that ultimately lead to mitochondrial dysfunction and caspase activation. These intrinsic events were not, however, the only mechanism by which ethanol was found to induce apoptosis. Specifically, it was determined that ethanol can also induce apoptosis through the involvement of the extrinsic death receptor pathway via the Fas/CD95 receptor system[50].
In other works, acute studies involving ethanol treatment to cells isolated from animals also demonstrated that oxidative stress was involved in mitochondrial membrane changes that were found to result in cytochrome c release and caspase activation[51,52]. Additionally, studies using developed recombinant cell lines demonstrated the role ethanol-inducible CYP2E1 has in alcohol-mediated apoptosis. Particularly, it was shown that ethanol-induced apoptosis was related to oxidative stress and subsequent lipid peroxidation in CYP2E1 over expressing cells[53], confirming the central role ROS and CYP2E1 have in ethanol-mediated cell death as hypothesized in previous works[25,54-56]. Additional evaluations demonstrated that such activation of intrinsic apoptotic pathways could be related to alcohol-mediated glutathione depletion which enhances the sensitivity of the cell to succumb to death, especially when faced with additional insults (e.g. increases in TGF-β expression or the presence of Hepatitis virus)[52,57-59]. Furthermore, continued searches for pro-apoptotic mechanisms involved in ethanol-mediated cell injury have provided additional information regarding the potential contribution regulatory mechanisms have in hepatocellular apoptosis, such as the role of NF-κB and ethanol-mediated alterations in proteosome function[60,61]; whether the processes are p53 dependent[62,63]; and what role pro-apoptotic Bcl-2 family proteins have in mediating mitochondrial permeability and apoptosis during alcohol cytotoxicity[49].
Overall, substantial information has been gained linking oxidative stress from alcohol metabolism via ADH and CYP2E1 to the induction of several pro-apoptotic mechanisms involving both intrinsic and extrinsic apoptosis pathways. However, the development and use of additional model systems, such as a recently described polarized hepatic cell line, may significantly contribute to our understanding of pro-apoptotic mechanisms induced as a consequence of ethanol administration.
ETHANOL-INDUCED APOPTOSIS IN A POLARIZED LIVER CELL MODEL
Many of the studies performed previously concerning the effect of ethanol on cellular processes have used alcohol-fed animal models as sources of isolated hepatocytes in order to provide more compatible models to human pathology than most cell culture systems provide. However, limitations were often observed in the model systems used. For example, the study of protein trafficking events in cultured systems has been hampered by the lack of a well-polarized cell that adequately mimics the complexity of in vivo hepatocyte protein delivery systems (i.e. the indirect transport of apical membrane proteins). Recently, our laboratory has demonstrated that the use of the WIF-B hepatoma hybrid cell line (in which polarity is a stable and dominant trait)[64,65] is an ideal in vitro model for studying the effects of ethanol on cellular processes[66]. Specifically, the WIF-B cells were found to exhibit alcohol dehydrogenase (ADH) activity allowing for the efficient metabolism of ethanol. Also, increases were observed in cellular triglyceride levels in the WIF-B cells following ethanol treatment, similar to the reported fat accumulation that is observed in human alcoholic liver injury. In addition, treatment of the WIF-B cells with alcohol resulted in morphological changes in the cells as demonstrated by decreases in bile canalicular formation and cell-cell contacts[66]. Thus, the use of the WIF-B cell line to study ethanol-induced cellular mechanisms is quite appropriate since the cells present a unique culture system that has the ability to metabolize ethanol and is a system that more closely resembles human morphology than other known cell lines.
The WIF-B hybrid cell is a cross between a human fibroblast (WI 38) and a Fao rat hepatoma cell line[64,67]. This clone represents a polarized and differentiated cell of hepatic origin that exhibits long-term viability in culture, develops a hepatocellular-polarized phenotype and expresses human genes coding for liver-specific proteins (albumin, fibrinogen, and ADH)[68]. One of the unique characteristics of the WIF-B clone is that the cells grow in monolayers and acquire a polarized phenotype as the cells form bile canalicular-like spaces (BC) that have concentrated apical membrane proteins. Therefore, these cells are useful for the functional studies of hepatocyte-specific properties such as the transport of membrane proteins. Indeed, several studies have utilized the advantages of the maintained polarized cell to study intercellular communication, bile acid transport systems, and membrane trafficking pathways[69-71].
In recent studies using this hepatic model, it was demonstrated that ethanol treatment induces apoptosis via signals emanating from the pro-apoptotic death receptor systems (i.e. Fas/CD95) as well as from intrinsic signals that resulted in mitochondrial changes[72]. Specifically, it was demonstrated that ethanol treatment of WIF-B cells resulted in a significant increase in apoptotic-related morphological changes (cells that contained condensed chromatin) that were also associated with pro-apoptotic biochemical features (indicated by the induction of caspase-3 activity). Also, it was shown that caspase-3 activation in ethanol-treated WIF-B cells was related to mitochondrial permeability transition (MPT) as ethanol treatment resulted in cytochrome c release that was found to be sensitive to cyclosporine A (an inhibitor of MPT). Additionally, it was determined that in the ethanol-induced apoptosis in WIF-B cells, the activities of upstream initiator caspases (caspase-2 and caspase-8), that are directly related to membrane signaling events of death receptors such as Fas, were increased. Concurrently, it was determined that Fas protein levels in the membrane fraction of the cell were found to be enhanced without a corresponding change in mRNA levels.
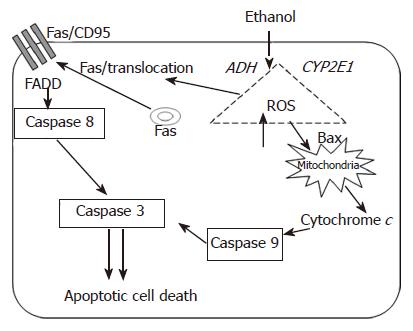
Overall, it was shown that ethanol treated WIF-B cells were induced to undergo apoptosis via protease activation and this was found to be related to death receptor signaling as well as mitochondrial stress events (Figure 1). These results are consistent with those reported in other model systems, especially the work by Minana et al[50]. In those studies, the investigators demonstrated that ROS (produced as a consequence of ethanol metabolism) induced apoptosis by two different mechanisms that involved MPT and the Fas receptor system. However, characterization of CYP2E1-mediated events in ethanol-mediated apoptosis in WIF-B cells has not been completed. In preliminary work, it has become evident that the WIF-B model system may be quite useful as CYP2E1 activity and protein levels can be reproducibly evaluated in these cells by ethanol as well as pyrazole treatment (unpublished data). Therefore, the utilization of WIF-B cells, a model which allows manipulation of the ethanol metabolizing systems, may significantly increase the potential of acquiring novel and clinically relevant information. Thus, further studies using WIF-B cells aim to enhance our understanding of crosstalk mechanisms that may exist between the major apoptotic-inducing pathways (extrinsic and intrinsic). Also, a more complete understanding of how apoptotic signals are favored and triggered over survival or compensatory signals is sought. Additionally, information as to how necrotic cell death mechanisms overcome apoptotic signals as the duration and amount of ethanol consumption is increased could potentially be determined using the WIF-B model system.
Figure 1 Schematic representation of the potential pathways involved in the initiation and propagation of ethanol-induced apoptosis.
Ethanol metabolism and the consequential generation of reactive oxygen species are implicated in the activation of pro-apoptotic mechanisms via death receptor (i.e. Fas/CD95) as well as intrinsic apoptotic pathways.
CONCLUSION
Apoptotic hepatotoxicity has been implicated in the pathogenesis of several liver diseases including that involving ethanol abuse. Insights into the cellular mechanisms involved in the initiation and propagation of apoptosis will significantly impact our understanding of alcohol-induced liver disease and may lead to the potential development of therapeutic interventions. The use of an emerging model system, polarized hepatic WIF-B cells, could significantly impact the study of alcohol-related hepatocellular injury, especially concerning the delineation of mechanisms involved in ethanol-induced cell death. In addition, polarized hepatic cell cultures may aid in the acquisition of translational information as the WIF-B cells offer a more compatible model system that better correlates to human pathology for analysis of potential therapeutic targets that could modulate apoptotic mechanisms induced by ethanol.
S- Editor Ma N L- Editor Alpini GD E- Editor Yin DH