Published online Oct 7, 2007. doi: 10.3748/wjg.v13.i37.4947
Revised: July 18, 2007
Accepted: July 26, 2007
Published online: October 7, 2007
Alcoholic liver disease is a major health care problem worldwide. Findings from many laboratories, including ours, have demonstrated that ethanol feeding impairs several of the many steps involved in methionine metabolism. Ethanol consumption predominantly results in a decrease in the hepatocyte level of S-adenosylmethionine and the increases in two toxic metabolites, homocysteine and S-adenosylhomocysteine. These changes, in turn, result in serious functional consequences which include decreases in essential methylation reactions via inhibition of various methyltransferases. Of particular interest to our laboratory is the inhibition of three important enzymes, phosphatidylethanolamine methyltransferase, isoprenylcysteine carboxyl methyltransferase and protein L-isoaspartate methyltransferase. Decreased activity of these enzymes results in increased fat deposition, increased apoptosis and increased accumulation of damaged proteins-all of which are hallmark features of alcoholic liver injury. Of all the therapeutic modalities available, betaine has been shown to be the safest, least expensive and most effective in attenuating ethanol-induced liver injury. Betaine, by virtue of aiding in the remethylation of homocysteine, removes both toxic metabolites (homocysteine and S-adenosylhomocysteine), restores S-adenosylmethionine level, and reverses steatosis, apoptosis and damaged proteins accumulation. In conclusion, betaine appears to be a promising therapeutic agent in relieving the methylation and other defects associated with alcoholic abuse.
- Citation: Kharbanda KK. Role of transmethylation reactions in alcoholic liver disease. World J Gastroenterol 2007; 13(37): 4947-4954
- URL: https://www.wjgnet.com/1007-9327/full/v13/i37/4947.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i37.4947
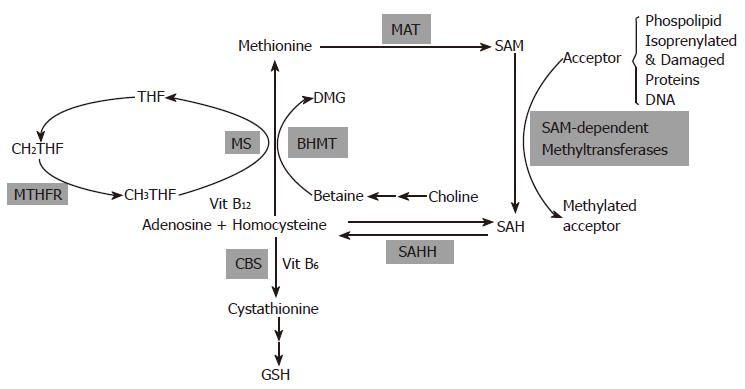
Biological transmethylation reactions utilize methyl groups derived from dietary methyl donors and from cofactors carrying 1-carbon units. A pathway that is central to many of these reactions is the metabolic cycling of methionine (Figure 1).
The significant role of methionine metabolism in liver is underscored by the fact that nearly half of the daily intake of methionine in humans and rats is metabolized there via methionine adenosyltransferase (MAT)-catalyzed reaction to generate S-adenosylmethionine (SAM), the principal methylating agent in the body. Under normal conditions, most of the 6-8 g SAM generated per day serves as a methyl donor for many transmethylation reactions. The normal estimated rate of these reactions, corrected to 70 kg body weight, is 16.7-23.4 mmol/d in young normal adults and 15.5-21.7 mmol/d in elderly subjects[1]. At least 50 of such SAM-dependent methylation reactions have been identified in mammals[2]. These reactions catalyzed by diverse methyltransferases result in the production of methylated biomolecules, such as phospholipids, proteins, nucleic acids and small molecules that have vital biological roles in biosynthesis, regulation, repair and detoxification. The other product is S-adenosylhomocysteine (SAH), which is hydrolyzed to adenosine and homocysteine by S-adenosylhomocysteine hydrolase (SAHH). This series of reactions is referred to as transmethylation. The methionine cycle is completed when homocysteine is remethylated back to methionine. The biochemical step between homocysteine and methionine occurs through two reactions, both of which are equally important in converting homocysteine to methionine in the liver[3]. In the first, a methyl group is transferred from betaine (which in turn, is derived from the oxidation of choline) to homocysteine to form methionine and dimethylglycine (DMG) via betaine-homocysteine methyltransferase (BHMT). The second reaction utilizes folate and through the action of methionine synthetase (MS), a methyl group is transferred from 5-methyltetrahydrofolate (MTHF) to vitamin B12 to form methylcobalamine. The methylcobalamine in turn transfers the methyl group to homocysteine to produce methionine. The remethylation of homocysteine by the folate pathway is ubiquitously distributed but the BHMT-mediated pathway is tissue-specific existing primarily in the liver and kidney[4]. Another enzyme that links the one-carbon pool and methionine metabolic pathway is 5, 10 methylene tetrahydrofolate reductase (MTHFR) that is involved in the production of MTHF. Folate also functions to transfer one carbon units for the synthesis of thymidylate, a key step in DNA synthesis.
Homocysteine can also be irreversibly catabolized through the transsulfuration pathway by the action of cystathionine β-synthase (CBS). The transsulfuration pathway superimposes on the methionine pathway and is important for the synthesis of cysteine and glutathione (GSH) as well as the removal of homocysteine. The transsulfuration occurs in a restricted number of mammalian tissues-liver, kidney, pancreas and the intestine, because the rest of the organs lack expression of one or more enzymes of this pathway.
Many laboratories have shown that alcohol consumption induces alterations in methionine metabolic pathways. A major defect elicited by ethanol consumption appears to be inhibition of liver MS activity, which results in impaired remethylation of homocysteine to form methionine[5-8]. Concomitantly, to compensate for the decreased MS activity, the activity of BHMT, an alternate enzyme for homocysteine remethylation, is induced after ethanol ingestion. A major outcome of this increase is a marked lowering in liver betaine levels in an effort to maintain SAM levels for transmethylation reactions[9]. However, SAM levels diminish upon prolonged ethanol exposure[10]. This does not appear to be due to a loss in liver-specific MAT level or activity since no decrease was observed despite 9 wk of intragastric feeding with ethanol and high fat[11]. Similar results were also reported for micropigs fed chronic ethanol for up to a year[7]. However, subsequent studies done by the same group have revealed decreased liver SAM levels in micropigs fed ethanol for 14 wk[12]. Data from human studies also demonstrates subnormal MAT function in liver biopsies from alcoholic patients[13,14].
Our laboratory and others have further reported that despite the compensatory increase in BHMT, alcohol-induced impairment of liver MS activity results in an enhanced generation of the potentially toxic agent homocysteine[12,15,16]. This in turn results in an increased intracellular level of SAH[7,8,12,17,18] as the equilibrium of the SAH hydrolase reaction energetically favors SAH generation over SAH hydrolysis. We further reported that the intracellular ratio of SAM:SAH levels was significantly lower (2.5) in freshly isolated hepatocytes and livers of ethanol-fed rats as compared to the ratio of 5 observed in hepatocytes and livers of control-fed rats [17,19]. Although many laboratories have shown comparable ratio values and decreases with ethanol as we report[20,21], studies with mice and micropigs reveal a similar trend of ratio decrease but lower absolute ratio values[22,23].
It is very crucial to maintain SAM:SAH ratios because SAH is a competitive inhibitor for many SAM-dependent methyltransferases. The Ki value for SAH is in the submicro-molar to low micromolar range and is often less than the Km value for SAM for many of the methyltransferases[24]. Therefore, any significant decrease in the ratio negatively affects many transmethylation reactions.
Ours and other laboratories have also shown that betaine (a naturally occurring tertiary amine, trimethylglycine) can enhance the remethylation of homocysteine via BHMT-catalyzed reaction[15,16] to alleviates ethanol-induced changes in intracellular SAH levels[17] as well as cause elevations in SAM levels[19,25]. Similar increases in hepatic SAM levels have also been reported in mice fed intragastrically an ethanol diet supplemented with betaine[26]. Betaine, by modulating SAM and SAH levels, results in normalization of the alcohol-induced alterations in the hepatocellular SAM:SAH ratios[17,19].
We have further observed that while ethanol treatment resulted in a 2-fold elevation of hepatic BHMT activity, this increase was further elevated in the livers of rats fed ethanol diet supplemented with 1% betaine. This increase in activity correlates with the enzyme level of BHMT quantified by western blot analysis using an antibody to rat BHMT as well as with quantitative RT-PCR studies[19]. These results imply that the inclusion of betaine in the diet promotes the alternate remethylation pathway to ultimately maintain hepatocellular SAM:SAH ratios by not only providing the substrate, betaine, for the BHMT-catalyzed reactions, but also further inducing BHMT at the gene and protein level. Further studies indicate an interplay between ethanol and not only the diet composition, but also its presentation[27].
Researchers over the years have attempted to determine which transmethylation reactions are affected by acute or chronic ethanol consumption as well as to understand how these altered reactions in turn, contribute to alcohol-induced liver injury. Further attempts have been made to determine the therapeutic effects of some participants of the methionine cycle, such as betaine and SAM, in minimizing the adverse effects.
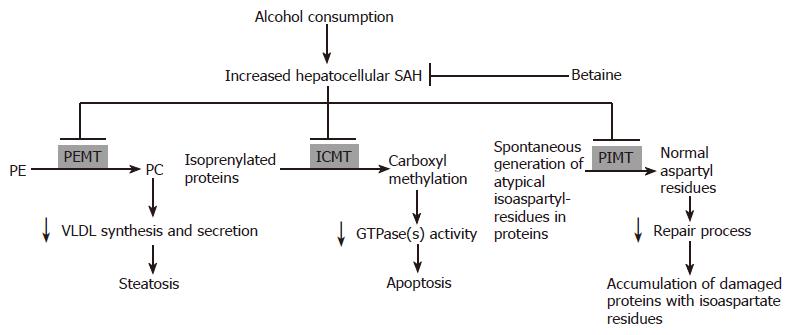
Our laboratories and others have shown that there are many SAM-dependent methylation reactions that are adversely affected by ethanol consumption and normalized by co-administration by betaine. Of particular interest to us is three such reactions that clearly demonstrates a close relationship between the activities of methyltransferases with hallmark features of alcoholic liver injury. These three liver methyltransferases are: (1) Phosphatidylethanolamine methyltransferase (PEMT), the methyltransferase involved in the generation of phosphatidylcholine (PC); (2) isoprenylcysteine carboxyl methyltransferase (ICMT), the methyltransferase that carboxyl methylates isoprenylated proteins; (3) Protein L-isoaspartate methyltransferase (PIMT), the methyltransferase that catalyzes the repair of isoaspartyl sites of spontaneously damaged proteins.
PEMT catalyzes three successive methyl group transfers from SAM to phosphatidylethanolamine (PE) to generate PC. This pathway has been reported to be responsible for about 50% of total SAM utilized[28]. Although PC can also be synthesized by the Kennedy Pathway in the liver[29], it has recently been shown that the PC derived via the PEMT pathway is preferentially used in the assembly of very low density lipoproteins (VLDL) and is necessary for its normal secretion[30]. As the liver exports triglycerides and cholesterol only as constituents of VLDL particles, any impairment in the processes of either the synthesis or export of VLDL particles could lead to fat accumulation within the hepatocyte. Indeed, experimentally inhibiting PC synthesis via the PE methylation pathway or targeted PEMT gene disruption has been shown to impair the incorporation of triglycerides into VLDL and reduce its secretion by hepatocytes[30,31]. Conversely, stable expression of PEMT has been shown to enhance this secretion[31]. The ultimate substantiation of the critical role of methylation reactions catalyzed by PEMT in the prevention of fat accumulation in hepatocytes is provided by the observations that homozygous PEMT knockout mice spontaneously develop hepatic steatosis despite adequate choline in the diet[32].
While earlier studies have implicated decreased hepatic VLDL secretion to play a role in the development of alcoholic steatosis[33], the association of PEMT activity with alcoholic liver injury was suggested by reports that identified decreased PEMT activities in livers of cirrhotics and a modulation of PEMT activities by altered SAM:SAH ratios[14,34,35]. However, the relationship between PEMT activity, SAM:SAH ratios and alcoholic steatosis became apparent after our observations that the SAM:SAH ratios in livers and hepatocytes of ethanol-fed rats is much lower than the ratios in livers and isolated hepatocytes of control diet fed rats[17,19]. Our further understanding of the relationship between ratios, PEMT activities and steatosis became evident from experiments conducted in our laboratory using liver microsomal fractions, isolated hepatocytes and in an in vivo rat model of ethanol abuse.
Using microsomal fractions isolated from liver of Purina-fed rats, PEMT activities measured in the presence of varying amounts of SAH (such that the ratio of SAM:SAH in the incubation mixture varied to mimic ratios seen in hepatocytes from ethanol, control or in vitro betaine-supplemented conditions) revealed increasing inhibitions of PEMT activity with decreases in the SAM:SAH ratio. A 30-40% inhibition of PEMT activity was observed when the SAM:SAH ratio was 2.5 (which corresponds to the intracellular ratio observed in isolated hepatocytes from ethanol-fed rats) as compared to the activity observed at the ratio of 5.0 (the ratio observed in the controls). A ratio as seen in the betaine-supplemented hepatocytes essentially resulted in restored enzyme activity[17]. Identical experiments were also performed on fractions prepared of hepatocytes obtained from both control and ethanol-fed rats. While similar activities were noticed in isolated microsomal fractions obtained from both cell types under basal conditions[36], the PEMT activities were also equally impaired by the decreasing SAM:SAH ratios. These results implied: (1) that the PEMT enzyme level appears to be unaffected by alcohol consumption, but it is the alcohol-induced decrease in the hepatocellular SAM to SAH content ratio that causes impaired PEMT activity, and (2) betaine, by virtue of its ability to increase the ratio, would restore (increase) the activity of PEMT.
To examine the physiological relevance of the direct inhibition of PEMT activity in isolated microsomal factions by increasing SAH amounts, the influence of ethanol on PC generation via the PEMT pathway was also analyzed. It was observed that ethanol feeding resulted in decreased PC production by a PEMT catalyzed reaction. Betaine either as in vitro supplementation to isolated hepatocytes obtained from ethanol-fed rats or fed to rats on ethanol diet corrected the ethanol-induced decrease in SAM:SAH ratios[19] and by normalizing the PC production via the PEMT-catalyzed reaction, significantly reduced fatty infiltration associated with ethanol consumption[19,36]. It has also been shown that the PC to PE ratio is a key regulator of cell membrane integrity and plays a role in progression of steatosis to steatohepatitis[37].
The substrates of ICMT are proteins that terminate with the CAAX motif (where C = cysteine, A = aliphatic amino acid, and X = undefined). Such proteins first undergo prenylation of the cysteine residues, followed by proteolytic cleavage of the AAX sequence. ICMT subsequently catalyzes the reversible carboxyl methylation of the newly exposed isoprenylcysteine using SAM as the methyl donor. This post-translational modification is required for the normal activity of proteins such as GTPases that play a crucial role in the signaling of many complex biological processes, including the prevention of apoptosis[38,39].
The functional importance of normal ICMT in preventing apoptosis is demonstrated in many studies. Apoptosis in cultured cells can be induced by inhibiting the carboxyl methylation of endogenous physiological substrates of ICMT[40-42] or by elevated intracellular SAH levels[43,44]. That SAH-induced apoptosis could be prevented by over-expression of ICMT emphasizes an involvement of impaired ICMT in the apoptotic cascade[41]. The physiological importance of ICMT is further reiterated by studies on homozygous ICMT knockout mice. These mice present with developmental defects of the liver that are so severe that virtually all of the knockout embryos die by mid-gestation[45], possibly due to liver agenesis/apoptosis[46]. Subsequent studies revealed that ICMT-catalyzed carboxyl methylation of its substrates, Ras and Rho GTPases, are very critical for prevention of caspase activation and programmed cell death[41].
It has been demonstrated that ethanol administration is linked to increased hepatocyte apoptosis in both clinical and experimental alcohol-induced liver injury in a variety of species of animals including rats, mice and minipigs[7,16,47-52]. Compelling evidence from our laboratory implicates altered hepatocellular SAM:SAH ratios and subsequent decreased ICMT activity to be responsible for alcoholic apoptosis. The first supporting data for this conjecture is that betaine supplementation (which normalizes homocysteine and hepatocellular SAM:SAH ratios) is effective in preventing hepatocyte apoptosis[53]. While Ji et al[16,26], using the mouse model of intragastric ethanol feeding, have also shown the protective effect of betaine on alcoholic apoptosis, their focus has been in pursuing a link of homocysteine-induced ER stress to apoptosis. But further studies in our laboratories using biochemical approaches have confirmed that indeed it is the increased intracellular SAH levels (that were achieved by using adenosine or tubercidin to specifically increase SAH levels without a change in homocysteine levels) that cause increases in apoptosis of hepatocytes. Further, these induced increases in apoptosis and increased SAH levels could both be significantly attenuated (40%-50%) by simultaneous betaine exposure[53]. Verification that the protective effect of betaine was primarily via the BHMT-catalyzed methyl group transfer, and not due to osmoregulation[54], was illustrated by using DMG[53], a product feedback inhibitor of BHMT[55].
We have also shown increasing inhibitions of ICMT activity with decreases in the SAM:SAH ratio similar to our observations with PEMT. Almost 30% less ICMT activity was observed when the SAM:SAH ratio was 2.5 (intracellular ratio in hepatocytes from ethanol-fed rats) as compared to the activity observed at the ratio of 5.0 (the ratio observed in the controls or betaine-fed ethanol rats). We have also shown that hepatocellular ICMT enzyme levels are not affected by ethanol consumption (Kharbanda and Tuma, unpublished observations). There is evidence that the ICMT activity may be enhanced in non-parenchymal hepatic cells after ethanol consumption[56,57], which suggests that the alterations in methylation reactions may be limited to alcohol-metabolizing cells.
Final support for a role of normal ICMT in preventing apoptosis has been derived from experiments using inhibitors of ICMT such as cysmethynil[58], N-acetyl-L-farnesyl cysteine (AFC) and N-acetyl-geranylgeranyl cysteine (AGGC). Data shows that exposure to cysmethynil, AFC or AGGC induce hepatocytes to undergo apoptosis (Kharbanda and Tuma, unpublished observations). These observations clearly implicate a role of normal carboxyl methylation of endogenous ICMT substrate(s) in hepatocytes to play crucial roles in the prevention of apoptosis as has been shown for other cell types[40-42]. Efforts are in progress to determine the downstream effector(s) of ICMT-catalyzed transmethylation reactions associated with inhibition of apoptosis of hepatocytes.
There are also reports that caspase-8 gene expression is sensitive to intracellular methylation status[59]. Alcohol-induced increased SAH levels could directly increase caspase-8 expression/activity to result in enhanced apoptosis[18].
Proteins undergo spontaneous thermodynamically driven changes over time that can result in decreased functionality. A common form of such damage is the spontaneous and non-enzymatic modification of aspartate and asparagine (which are among the most unstable residues in proteins and peptides) to form atypical isoaspartyl residues. The enzyme PIMT catalyzes the transfer of the methyl group of SAM to these isoaspartyl sites, allowing reisomerization and restoration of the original alpha peptide linkage after repeated rounds of methylation[60]. This physiological repair reaction helps prevent the accumulation of the protein molecules containing these abnormal aspartyl residues. In mammals, PIMT is expressed in all tissues with the highest level in the brain and blood tissues[61]. Homozygous PIMT knockout mice accumulate damaged proteins in their tissues and organs, including the liver[61].
Isoaspartate residues have been identified in a variety of cellular structural and functional proteins such as tubulin, calmodulin, histone 2B and collagen type-I. Evidence has been accumulating that the modified proteins have impaired biological activity that can be substantially restored via methylation by PIMT[62]. The physiological relevance of this repair by PIMT has also been demonstrated in vivo by studies using PIMT-deficient mice. These animals show aberrant vesicular transport which is caused by microtubule disorganization resulting from increased amounts of isoaspartyl residues in β-tubulin, a major substrate of PIMT and a component of microtubules[63].
Environmental factors such as heat shock, photochemical stress and oxidative stress have been reported to result in an enhanced accumulation of damaged proteins with isoaspartate residues[64,65].
Our recent data shows that in addition to the above mentioned factors, chronic alcohol ingestion results in the accumulation of isoaspartyl-bearing damaged proteins in rat liver homogenates, particularly in the cytosol and microsomal fractions. This accumulation correlates to the lower SAM:SAH ratios and consequent impaired PIMT activity in livers of these rats as no change in the PIMT enzyme level by alcohol was observed. Furthermore, rats fed the betaine-supplemented ethanol diet showed similar isoaspartyl residue quantities per unit protein as controls. This restoration of the protein repair process was related to the effect of betaine to normalize the SAM:SAH ratios, which in turn, normalizes the PIMT-catalyzed protein repair methylation reaction[66].
We also determined the effect of varying SAM:SAH ratios on the activity of purified PIMT. We observed 30% less PIMT activity at a ratio of 2.5 (which corresponds to the ratio in livers of ethanol-fed rats) as compared to a ratio of 5 as seen in livers of control or betaine-supplemented fed ethanol rats[66]. These results were similar to those obtained with PEMT and ICMT activity measurements.
We are currently focusing on identifying some of the unique cellular structural and functional proteins susceptible to isoaspartyl damage following ethanol consumption by a proteomic approach and in understanding their role in altering the hepatocellular dynamics that could contribute to the progression of alcoholic liver injury.
DNA Methyltransferases and Alcohol: Modulation of gene expression by alterations in DNA methylation can result in epigenetic changes and functional consequences. Current investigations are focused in understanding the molecular steps involved in the effects of ethanol on epigenetic modifications in relation to pathological changes in the liver. The inhibition of SAM-dependent DNA methyltransferase activities by ethanol-induced decreased SAM:SAH ratio could be responsible for the impaired DNA methylation reported in rats fed intragastrically with ethanol and a high fat diet[11]. The reduced hepatic DNA methylation patterns by ethanol consumption has been reported to be associated with increased expression of c-myc and increased genome-wide DNA strand break accumulation[11]. Other studies have shown no effects of alcohol consumption on O6-methylguanine DNA methyltransferase levels in the liver of cirrhotic[67], however its activity may be affected by altered SAM:SAH ratios. More information can be obtained from an excellent editorial of the epigenetic effects of ethanol on liver injury that was recently published[68].
Glycine-N-Methyltransferase and Alcohol: This is an abundant cytosolic SAM-dependent methyltransferase involved in the synthesis of sarcosine. But GNMT, unlike PEMT, ICMT, PEMT and the DNA methyltransferases, is less sensitive to inhibition by SAH[24]. However, it is allosterically inhibited by MTHF[69] and plays a role in regulating SAM:SAH ratios[70]. Ethanol has been shown to decrease GNMT transcript levels, however an increase in its activity was observed after alcohol consumption, possibly related to decreased MTHF levels[71].
Alcohol-induced elevation of intracellular SAH levels can impair the methylation reactions catalyzed by three enzymes, PIMT, PEMT and ICMT, which consequently contribute to the accumulation of damaged proteins with isoaspartyl residues and the induction of hepatic steatosis and apoptosis, respectively. We further provide data to support that these pathologies can be significantly attenuated by betaine administration. We believe that this protection is via remethylation of homocysteine that leads to normalized intracellular SAH levels and maintenance of normal methylation reactions of these three and other methyltransferases. A schematic of this working scheme is depicted as Figure 2. Overall, we have identified that the increased hepatocellular SAH levels generated by chronic ethanol exposure can negatively affect the activities of the three methyltransferases, PEMT, ICMT and PIMT These methylation defects, in turn, cause decreased synthesis and secretion of VLDL, impaired activation of GTPases and diminished protein repair process on molecules such as tubulin. These deficiencies ultimately contribute to the induction of steatosis, apoptosis and the accumulation of damaged proteins which could exaggerate hepatocellular injury by disrupting normal cellular function including trafficking events. Betaine administration can prevent the increase in SAH and thereby prevent these ethanol-induced pathologies.
In addition to its effects in ameliorating alcoholic liver injury, betaine has also been shown to prevent carbon tetrachloride-induced liver injury[72] as well as to considerably decrease indices of steatosis in nonalcoholic steatohepatitis (NASH) patients[73-75]. These properties along with the low cost and easy use and availability of betaine underscore the advantages of choosing this metabolite for the treatment of liver injury.
S- Editor Ma N L- Editor Alpini GD E-Editor Li JL
| 1. | Mudd SH, Brosnan JT, Brosnan ME, Jacobs RL, Stabler SP, Allen RH, Vance DE, Wagner C. Methyl balance and transmethylation fluxes in humans. Am J Clin Nutr. 2007;85:19-25. [PubMed] |
| 2. | Schubert HL, Blumenthal RM, Cheng X. Many paths to methyltransfer: a chronicle of convergence. Trends Biochem Sci. 2003;28:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 709] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 3. | Finkelstein JD, Martin JJ. Methionine metabolism in mammals. Distribution of homocysteine between competing pathways. J Biol Chem. 1984;259:9508-9513. [PubMed] |
| 4. | Delgado-Reyes CV, Wallig MA, Garrow TA. Immunohistochemical detection of betaine-homocysteine S-methyltransferase in human, pig, and rat liver and kidney. Arch Biochem Biophys. 2001;393:184-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Barak AJ, Baker H, Tuma DJ. Influence of Ethanol on In-Vivo Levels of Hepatic Methylators Betaine and N5- Methyltetrahydrofolate in the Rat. IRCS Med Sci. 1981;9:527-528. |
| 6. | Barak AJ, Beckenhauer HC, Tuma DJ. Ethanol feeding inhibits the activity of N5-methyltetrahydrofolate-homocysteine methyltransferase in the rat. IRCS Med Sci. 1985;13:760-761. |
| 7. | Halsted CH, Villanueva J, Chandler CJ, Stabler SP, Allen RH, Muskhelishvili L, James SJ, Poirier L. Ethanol feeding of micropigs alters methionine metabolism and increases hepatocellular apoptosis and proliferation. Hepatology. 1996;23:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 124] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Trimble KC, Molloy AM, Scott JM, Weir DG. The effect of ethanol on one-carbon metabolism: increased methionine catabolism and lipotrope methyl-group wastage. Hepatology. 1993;18:984-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Barak AJ, Beckenhauer HC, Tuma DJ, Donohue TM. Adaptive increase in betaine-homocysteine methyltransferase activity maintains hepatic S-adenosylmethionine levels in ethanol-treated rats. IRCS Med Sci. 1984;12:866-867. |
| 10. | Barak AJ, Beckenhauer HC, Tuma DJ, Badakhsh S. Effects of prolonged ethanol feeding on methionine metabolism in rat liver. Biochem Cell Biol. 1987;65:230-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 115] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Lu SC, Huang ZZ, Yang H, Mato JM, Avila MA, Tsukamoto H. Changes in methionine adenosyltransferase and S-adenosylmethionine homeostasis in alcoholic rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;279:G178-G185. [PubMed] |
| 12. | Halsted CH, Villanueva JA, Devlin AM, Niemelä O, Parkkila S, Garrow TA, Wallock LM, Shigenaga MK, Melnyk S, James SJ. Folate deficiency disturbs hepatic methionine metabolism and promotes liver injury in the ethanol-fed micropig. Proc Natl Acad Sci U S A. 2002;99:10072-10077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 149] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Cabrero C, Duce AM, Ortiz P, Alemany S, Mato JM. Specific loss of the high-molecular-weight form of S-adenosyl-L-methionine synthetase in human liver cirrhosis. Hepatology. 1988;8:1530-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 133] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Duce AM, Ortíz P, Cabrero C, Mato JM. S-adenosyl-L-methionine synthetase and phospholipid methyltransferase are inhibited in human cirrhosis. Hepatology. 1988;8:65-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 228] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Barak AJ, Beckenhauer HC, Kharbanda KK, Tuma DJ. Chronic ethanol consumption increases homocysteine accumulation in hepatocytes. Alcohol. 2001;25:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 448] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 17. | Barak AJ, Beckenhauer HC, Mailliard ME, Kharbanda KK, Tuma DJ. Betaine lowers elevated s-adenosylhomocysteine levels in hepatocytes from ethanol-fed rats. J Nutr. 2003;133:2845-2848. [PubMed] |
| 18. | Song Z, Zhou Z, Uriarte S, Wang L, Kang YJ, Chen T, Barve S, McClain CJ. S-adenosylhomocysteine sensitizes to TNF-alpha hepatotoxicity in mice and liver cells: a possible etiological factor in alcoholic liver disease. Hepatology. 2004;40:989-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Kharbanda KK, Mailliard ME, Baldwin CR, Beckenhauer HC, Sorrell MF, Tuma DJ. Betaine attenuates alcoholic steatosis by restoring phosphatidylcholine generation via the phosphatidylethanolamine methyltransferase pathway. J Hepatol. 2007;46:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 20. | Choi SW, Stickel F, Baik HW, Kim YI, Seitz HK, Mason JB. Chronic alcohol consumption induces genomic but not p53-specific DNA hypomethylation in rat colon. J Nutr. 1999;129:1945-1950. [PubMed] |
| 21. | Ubeda N, Alonso-Aperte E, Varela-Moreiras G. Acute valproate administration impairs methionine metabolism in rats. J Nutr. 2002;132:2737-2742. [PubMed] |
| 22. | Lu SC, Alvarez L, Huang ZZ, Chen L, An W, Corrales FJ, Avila MA, Kanel G, Mato JM. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci U S A. 2001;98:5560-5565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 353] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 23. | Esfandiari F, Villanueva JA, Wong DH, French SW, Halsted CH. Chronic ethanol feeding and folate deficiency activate hepatic endoplasmic reticulum stress pathway in micropigs. Am J Physiol Gastrointest Liver Physiol. 2005;289:G54-G63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Clarke S, Banfield K. S-adenosylmethionine-dependent methyltransferases. Homocysteine in health and disease. Cambridge: Cambridge University Press 2001; 63-78. |
| 25. | Barak AJ, Beckenhauer HC, Junnila M, Tuma DJ. Dietary betaine promotes generation of hepatic S-adenosylmethionine and protects the liver from ethanol-induced fatty infiltration. Alcohol Clin Exp Res. 1993;17:552-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 125] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Ji C, Deng Q, Kaplowitz N. Role of TNF-alpha in ethanol-induced hyperhomocysteinemia and murine alcoholic liver injury. Hepatology. 2004;40:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Pajares MA, Pérez-Sala D. Betaine homocysteine S-methyltransferase: just a regulator of homocysteine metabolism? Cell Mol Life Sci. 2006;63:2792-2803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Noga AA, Stead LM, Zhao Y, Brosnan ME, Brosnan JT, Vance DE. Plasma homocysteine is regulated by phospholipid methylation. J Biol Chem. 2003;278:5952-5955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Kennedy EP, Weiss SB. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956;222:193-214. [PubMed] |
| 30. | Nishimaki-Mogami T, Yao Z, Fujimori K. Inhibition of phosphatidylcholine synthesis via the phosphatidylethanolamine methylation pathway impairs incorporation of bulk lipids into VLDL in cultured rat hepatocytes. J Lipid Res. 2002;43:1035-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Noga AA, Zhao Y, Vance DE. An unexpected requirement for phosphatidylethanolamine N-methyltransferase in the secretion of very low density lipoproteins. J Biol Chem. 2002;277:42358-42365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Zhu X, Song J, Mar MH, Edwards LJ, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) knockout mice have hepatic steatosis and abnormal hepatic choline metabolite concentrations despite ingesting a recommended dietary intake of choline. Biochem J. 2003;370:987-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Venkatesan S, Ward RJ, Peters TJ. Effect of chronic ethanol feeding on the hepatic secretion of very-low-density lipoproteins. Biochim Biophys Acta. 1988;960:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Lieber CS, Robins SJ, Leo MA. Hepatic phosphatidylethanolamine methyltransferase activity is decreased by ethanol and increased by phosphatidylcholine. Alcohol Clin Exp Res. 1994;18:592-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 71] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Hoffman DR, Haning JA, Cornatzer WE. Microsomal phosphatidylethanolamine methyltransferase: inhibition by S-adenosylhomocysteine. Lipids. 1981;16:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Kharbanda KK, Rogers DD, Mailliard ME, Siford GL, Barak AJ, Beckenhauer HC, Sorrell MF, Tuma DJ. A comparison of the effects of betaine and S-adenosylmethionine on ethanol-induced changes in methionine metabolism and steatosis in rat hepatocytes. J Nutr. 2005;135:519-524. [PubMed] |
| 37. | Li Z, Agellon LB, Allen TM, Umeda M, Jewell L, Mason A, Vance DE. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 2006;3:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 569] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 38. | Rando RR. Chemical biology of isoprenylation/methylation. Biochem Soc Trans. 1996;24:682-687. [PubMed] |
| 39. | Bergo MO, Leung GK, Ambroziak P, Otto JC, Casey PJ, Young SG. Targeted inactivation of the isoprenylcysteine carboxyl methyltransferase gene causes mislocalization of K-Ras in mammalian cells. J Biol Chem. 2000;275:17605-17610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 129] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Ratter F, Gassner C, Shatrov V, Lehmann V. Modulation of tumor necrosis factor-alpha-mediated cytotoxicity by changes of the cellular methylation state: mechanism and in vivo relevance. Int Immunol. 1999;11:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Kramer K, Harrington EO, Lu Q, Bellas R, Newton J, Sheahan KL, Rounds S. Isoprenylcysteine carboxyl methyltransferase activity modulates endothelial cell apoptosis. Mol Biol Cell. 2003;14:848-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Pérez-Sala D, Gilbert BA, Rando RR, Cañada FJ. Analogs of farnesylcysteine induce apoptosis in HL-60 cells. FEBS Lett. 1998;426:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Zhao Z, Kapoian T, Shepard M, Lianos EA. Adenosine-induced apoptosis in glomerular mesangial cells. Kidney Int. 2002;61:1276-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Dawicki DD, Chatterjee D, Wyche J, Rounds S. Extracellular ATP and adenosine cause apoptosis of pulmonary artery endothelial cells. Am J Physiol. 1997;273:L485-L494. [PubMed] |
| 45. | Bergo MO, Leung GK, Ambroziak P, Otto JC, Casey PJ, Gomes AQ, Seabra MC, Young SG. Isoprenylcysteine carboxyl methyltransferase deficiency in mice. J Biol Chem. 2001;276:5841-5845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 46. | Lin X, Jung J, Kang D, Xu B, Zaret KS, Zoghbi H. Prenylcysteine carboxylmethyltransferase is essential for the earliest stages of liver development in mice. Gastroenterology. 2002;123:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Benedetti A, Marucci L. The significance of apoptosis in the liver. Liver. 1999;19:453-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Higuchi H, Kurose I, Kato S, Miura S, Ishii H. Ethanol-induced apoptosis and oxidative stress in hepatocytes. Alcohol Clin Exp Res. 1996;20:340A-346A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Nanji AA. Apoptosis and alcoholic liver disease. Semin Liver Dis. 1998;18:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 50. | Casey CA, Nanji A, Cederbaum AI, Adachi M, Takahashi T. Alcoholic liver disease and apoptosis. Alcohol Clin Exp Res. 2001;25:49S-53S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Deaciuc IV, D'Souza NB, de Villiers WJ, Burikhanov R, Sarphie TG, Hill DB, McClain CJ. Inhibition of caspases in vivo protects the rat liver against alcohol-induced sensitization to bacterial lipopolysaccharide. Alcohol Clin Exp Res. 2001;25:935-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Ji C, Kaplowitz N. Hyperhomocysteinemia, endoplasmic reticulum stress, and alcoholic liver injury. World J Gastroenterol. 2004;10:1699-1708. [PubMed] |
| 53. | Kharbanda KK, Rogers DD, Mailliard ME, Siford GL, Barak AJ, Beckenhauer HC, Sorrell MF, Tuma DJ. Role of elevated S-adenosylhomocysteine in rat hepatocyte apoptosis: protection by betaine. Biochem Pharmacol. 2005;70:1883-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Craig SA. Betaine in human nutrition. Am J Clin Nutr. 2004;80:539-549. [PubMed] |
| 55. | Garrow TA. Purification, kinetic properties, and cDNA cloning of mammalian betaine-homocysteine methyltransferase. J Biol Chem. 1996;271:22831-22838. [PubMed] |
| 56. | Reif S, Aeed H, Shilo Y, Reich R, Kloog Y, Kweon YO, Bruck R. Treatment of thioacetamide-induced liver cirrhosis by the Ras antagonist, farnesylthiosalicylic acid. J Hepatol. 2004;41:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol. 2006;79:1348-1356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 58. | Winter-Vann AM, Baron RA, Wong W, dela Cruz J, York JD, Gooden DM, Bergo MO, Young SG, Toone EJ, Casey PJ. A small-molecule inhibitor of isoprenylcysteine carboxyl methyltransferase with antitumor activity in cancer cells. Proc Natl Acad Sci U S A. 2005;102:4336-4341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 59. | Attwood JT, Yung RL, Richardson BC. DNA methylation and the regulation of gene transcription. Cell Mol Life Sci. 2002;59:241-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 282] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 60. | Shimizu T, Matsuoka Y, Shirasawa T. Biological significance of isoaspartate and its repair system. Biol Pharm Bull. 2005;28:1590-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 61. | Kim E, Lowenson JD, MacLaren DC, Clarke S, Young SG. Deficiency of a protein-repair enzyme results in the accumulation of altered proteins, retardation of growth, and fatal seizures in mice. Proc Natl Acad Sci U S A. 1997;94:6132-6137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 229] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 62. | Johnson BA, Langmack EL, Aswad DW. Partial repair of deamidation-damaged calmodulin by protein carboxyl methyltransferase. J Biol Chem. 1987;262:12283-12287. [PubMed] |
| 63. | Lanthier J, Bouthillier A, Lapointe M, Demeule M, Béliveau R, Desrosiers RR. Down-regulation of protein L-isoaspartyl methyltransferase in human epileptic hippocampus contributes to generation of damaged tubulin. J Neurochem. 2002;83:581-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Ingrosso D, D'angelo S, di Carlo E, Perna AF, Zappia V, Galletti P. Increased methyl esterification of altered aspartyl residues in erythrocyte membrane proteins in response to oxidative stress. Eur J Biochem. 2000;267:4397-4405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 65. | D'Angelo S, Ingrosso D, Perfetto B, Baroni A, Zappia M, Lobianco LL, Tufano MA, Galletti P. UVA irradiation induces L-isoaspartyl formation in melanoma cell proteins. Free Radic Biol Med. 2001;31:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Kharbanda KK, Mailliard ME, Baldwin CR, Sorrell MF, Tuma DJ. Accumulation of proteins bearing atypical isoaspartyl residues in livers of alcohol-fed rats is prevented by betaine administration: effects on protein-L-isoaspartyl methyltransferase activity. J Hepatol. 2007;46:1119-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Miyakawa H, Liu J, Noguchi O, Marumo F, Sato C. Effect of alcohol drinking on gene expression of hepatic O6-methylguanine DNA methyltransferase in chronic liver diseases. Alcohol Clin Exp Res. 1996;20:297A-300A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 68. | Shukla SD, Aroor AR. Epigenetic effects of ethanol on liver and gastrointestinal injury. World J Gastroenterol. 2006;12:5265-5271. [PubMed] |
| 69. | Wagner C, Decha-Umphai W, Corbin J. Phosphorylation modulates the activity of glycine N-methyltransferase, a folate binding protein. In vitro phosphorylation is inhibited by the natural folate ligand. J Biol Chem. 1989;264:9638-9642. [PubMed] |
| 70. | Schalinske KL, Nieman KM. Disruption of methyl group metabolism by ethanol. Nutr Rev. 2005;63:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 71. | Villanueva JA, Halsted CH. Hepatic transmethylation reactions in micropigs with alcoholic liver disease. Hepatology. 2004;39:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 72. | Junnila M, Barak AJ, Beckenhauer HC, Rahko T. Betaine reduces hepatic lipidosis induced by carbon tetrachloride in Sprague-Dawley rats. Vet Hum Toxicol. 1998;40:263-266. [PubMed] |
| 73. | Abdelmalek MF, Angulo P, Jorgensen RA, Sylvestre PB, Lindor KD. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: results of a pilot study. Am J Gastroenterol. 2001;96:2711-2717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 290] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 74. | Miglio F, Rovati LC, Santoro A, Setnikar I. Efficacy and safety of oral betaine glucuronate in non-alcoholic steatohepatitis. A double-blind, randomized, parallel-group, placebo-controlled prospective clinical study. Arzneimittelforschung. 2000;50:722-727. [PubMed] |
| 75. | Mukherjee S, Bernard T, Schafer D, Barak AJ, Sorrell MF, Tuma DJ. Impact of betaine on hepatic fibrosis and homocysteine in non-alcoholic steatohepatitis-A prospective cohort study. Hepatology. 2005;42:610A. |