Published online Sep 14, 2007. doi: 10.3748/wjg.v13.i34.4615
Revised: June 22, 2007
Accepted: June 25, 2007
Published online: September 14, 2007
AIM: To investigate the inhibitory effect of kallistatin (KAL) on angiogenesis and HCT-116 xenograft tumor growth.
METHODS: Heterotopic tumors were induced by subcutaneous injection of 2 × 106 HCT-11 cells in mice. Seven days later, 2 × 1011 rAAV-GFP or rAAV-KAL was injected intratumorally (n = 5 for each group). The mice were sacrificed at d 28, by which time the tumors in the rAAV-GFP group had grown to beyond 5% of the total body weight. Tumor growth was measured by calipers in two dimensions. Tumor angiogenesis was determined with tumor microvessel density (MVD) by immunohistology. Tumor cell proliferation was assessed by Ki-67 staining.
RESULTS: Intratumor injection of rAAV-KAL inhibited tumor growth in the treatment group by 78% (171 ± 52 mm3) at d 21 after virus infection compared to the control group (776 ± 241 mm3). Microvessel density was significantly inhibited in tumor tissues treated with rAAV-KAL. rAAV-KAL also decreased the proportion of proliferating cells (Ki-67 positive cells) in tumors compared with the control group.
CONCLUSION: rAAV-mediated expression of KAL inhibits the growth of colon cancer by reducing angiogenesis and proliferation of tumor cells, and may provide a promising anti-angiogenesis-based approach to the treatment of metastatic colorectal cancer.
- Citation: Diao Y, Ma J, Xiao WD, Luo J, Li XY, Chu KW, Fung PW, Habib N, Farzaneh F, Xu RA. Inhibition of angiogenesis and HCT-116 xenograft tumor growth in mice by kallistatin. World J Gastroenterol 2007; 13(34): 4615-4619
- URL: https://www.wjgnet.com/1007-9327/full/v13/i34/4615.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i34.4615
Advanced colorectal cancer (CRC) is a critical health concern in the world; overall survival is highly dependent upon the stage of disease at diagnosis. The estimated 5-year survival rates range from 85% to 90% for patients with stageIdisease to < 5% for patients with stage IV disease. Over 50% of patients with colorectal cancer presenting with metastatic or locally advanced disease experience local recurrence or develop distant metastases after potentially curative surgery. The most important treatment currently available for patients with stage IV disease is systemic chemotherapy. Although there have been recent advances in the field, with randomized trials confirming the activity of irinotecan, oxaliplatin and capecitabine, median survival remains at only 15-18 mo. There is, therefore, a critical need for more effective and better-tolerated therapies.
With the role of angiogenesis in tumor growth and progression firmly established, considerable efforts have been directed to antiangiogenic therapy as a new modality to treat human cancers. Clinical trials have provided strong evidence that antiangiogenic agents, such as bevacizumab (avastin, anti-VEGF humanized monoclonal antibody), may play a meaningful role in colorectal anticancer therapy, with an optimistic increase of 20%-30% in survival. Based upon the results of these randomized studies[1,2], bevacizumab has now been approved by the FDA for the first-line treatment of metastatic colorectal cancer in combination with chemotherapy.
Despite the enthusiasm and promising early results, there are still several problems to resolve in evaluating the activity of antiangiogenic agents, which are predominantly cystostatic rather than cytotoxic, and the clinical results are still disappointing according to internationally accepted RECIST criteria. Antiangiogenic gene therapy strategy holds great promise in advancing antiangiogenesis as an effective cancer therapy to be evaluated in clinical trials in the future. Several lessons can be learned from early clinical trials in antiangiogenic therapy. (1) Prolonged use of angiogenesis inhibitors is envisioned for cancer patients. Because antiangiogenic agents stabilize tumor growth but do not reduce tumor burden, constitutive expression of an antiangiogenic protein even at lower concentrations than bolus doses may be more effective than the intermittent peaks associated with repeated delivery of a recombinant protein. Preclinical experiments have shown that a constant level of these inhibitors in the circulation provides more effective anti-cancer therapy than intermittent peaks of inhibitor in mice[3]. Therefore, in the future, antiangiogenic gene therapy may be important for protein angiogenesis inhibitors. (2) The angiogenic switch has become recognized as a critical step in tumor propagation and progression[4]. Multiple angiogenic pathways are involved in the balance between endogenous stimulators and inhibitors. From this perspective, the body may harbor many in situ tumors, yet the tumors do not progress to lethal tumors unless there is an imbalance between a tumor’s pro-angiogenic output and the body’s total angiogenic defense[5]. Gene therapy offers a strategy whereby an individual could boost their endogenous angiogenic defenses and tip the balance favorably, because multiple therapeutic genes can be engineered into one vector. (3) The production of functional proteins can be expensive, and repeated usages will not be affordable for patients. Gene therapy offers the opportunity for patients to become their own source of production, i.e., an endogenous factory for antiangiogenic protein production.
Among the identified endogenous inhibitors of angiogenesis, kallistatin (KAL) is one of the best choices because of its broad-spectrum characteristics[6]. It is capable of inhibiting vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) mediated angiogenesis [unpublished data], as well as preventing tumor invasion via the activation of metalloproteinases by inhibiting tissue kallikrein activity.
Gene transfer vectors based on adeno-associated virus (AAV) are of particular interest as they are capable of inducing transgene expression in a broad range of tissues for a relatively long time without stimulation of a cell-mediated immune response. Perhaps the most important attribute of AAV vectors is their safety profile in phaseIclinical trials ranging from cystic fibrosis (CF) to Parkinson’s disease. The utility of AAV vectors as a gene delivery agent in cancer therapy is showing promise in preclinical studies. With the identification of different serotypes and recent progress in the improvement of AAV vectors, such as dual vectors to overcome the limited packaging capacity, self-complementary vectors to increase the level and onset of transgene expression, and capsid modifications to mediate cell specific transduction, it will be possible in the future to design more specific and efficient therapies for use in the cancer treatment arena[7]. Therefore, an approach whereby the KAL gene is delivered to tumors in a form enabling stable and long-term gene expression has become increasingly attractive. Our recent laboratory work revealed that KAL could be a suitable candidate for hepatocellular carcinoma (HCC) therapy [unpublished data]. In the present study, an anti-angiogenic approach by transfer of the KAL gene through an AAV vector was employed to treat colon cancer in a mouse model.
The full-length cDNA fragments of human KAL were amplified from human liver first-stranded cDNA by PCR. Specific primers were designed from the nucleotide sequence of human KAL published in NCBI (accession number L19684), Kalli-F (5’-AAGAATTCGAGGATGCATCTTATCGAC) and Kalli-R (5’-AAGGTACCAAGCTTCTATGGTTTCGTGGGGTC). Restriction enzyme sites (underlined) were introduced into primers for subcloning. The conditions of PCR were 45 s each at 94°C, 50°C and 68°C for 36 cycles. The PCR fragments were sequenced and subcloned into the AAV-2 vector, which has been described previously[8,9]. The cDNA fragments of KAL were generated by PCR and were confirmed by DNA sequencing. The sequence of KAL matched that published in NCBI except for two nucleotides. The differences were at nucleotides 1145 and 1146, which resulted in a sense mutation in amino acid sequence. The residue threonine (ACG) at codon 382 was changed into a serine (AGC) residue.
To attain a constitutive and high-level expression of KAL, the KAL cDNA was inserted into the AAV vector between the inverted terminal repeats (ITRs) under the control of cytomegalovirus (CMV) enhancer/chicken β-actin promoter. A woodchuck hepatitis B virus post-transcriptional regulatory element (WPRE) was inserted into constructs immediately after the inserted genes, in order to boost transgene expression[10].
AAV particles were generated by a three plasmid, helper-virus free packaging method[8,15]. The viral titre was determined by real-time PCR analysis as described previously[11].
Fifty-thousand human embryonic kidney (HEK) 293 cells were seeded into 6-well plates and were grown overnight. The medium was replaced by complete medium with reduced fetal bovine serum (FBS) (2%). A total of 5 × 109 vector genome rAAV-GFP particles were incubated with cells for 8 h. Two days later, the ability of the virus to infect and transduce the cell line was assessed by fluorescent microscopy.
The HEK 293 cell line and the colon adenocarcinoma cell line HCT-116 were purchased from American Type Culture Collection (ATCC). The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen, Grand Island, NY) supplemented with 10% FBS (Gemini, Sacramento, California), 100 unit/mL penicillin and 100 μg/mL streptomycin (Invitrogen). Six- to eight-week-old male BALB/c mice were obtained from the Laboratory Animal Unit of the University of Hong Kong. All surgical procedures and care administered to the animals were approved by the University Ethics Committee and performed according to institutional guidelines. The anti-CD34 (clone MEC 14.7), anti-Ki-67 (clone B56) and anti-rat polyclonal antibodies and anti-mouse polymer conjugate were purchased from Santa Cruz (Santa Cruz, CA), Pharmingen (San Jose, CA), BD Biosciences (San Jose, CA) and Zymed (South San Francisco, CA), respectively.
Tumors were established by subcutaneous inoculation of 2 × 106 HCT-116 cells into the dorsal skin of mice using 25-G needles. Seven days later, 2 × 1011 rAAV-GFP or rAAV-KAL was injected intratumorally (n = 5 for each group). The mice were sacrificed at d 28, by which time the tumors in the AAV-GFP group had grown to beyond 5% of the total body weight.
Tumor growth was monitored for 4 wk by measuring two perpendicular diameters. Tumor volume was calculated according to the formula 0.52 ×a×b2, where a and b are the largest and smallest diameters, respectively.
Microvessel density (MVD) was assessed by the method defined by Weinder and co-workers[12] after CD34 staining. The mean value of the three hot spots was taken as the MVD, which was expressed as the absolute number of microvessels (0.7386 mm2 per field).
Positive and negative stained cells were counted on a minimum of 10 randomly selected × 400 high-power fields from representative sections of tumors. The Ki-67 proliferation index (the fraction of proliferating cells) was calculated from the number of Ki-67 positive cells divided by the total cell count.
Comparisons of tumor volume between groups were made with the Student’s t-test where indicated and were considered statistically significant if the P value was less than 0.05.
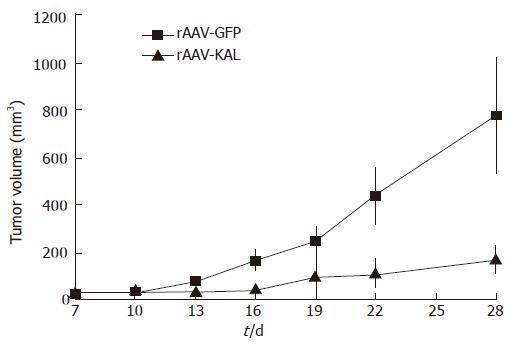
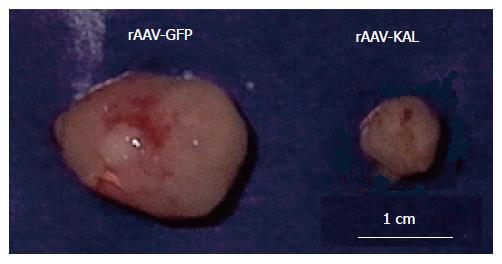
Tumor formation was detected in all of the mice. Tumor growth was significantly slower in the rAAV-KAL group than in animals injected with rAAV-GFP (Figure 1). At d 21 after virus infection, tumor growth was reduced by 78% (171 ± 52 mm3) in the treatment group compared to the control group (776 ± 241 mm3, P < 0.01). Representative photographs of the tumor at 21 d for both groups are shown in Figure 2.
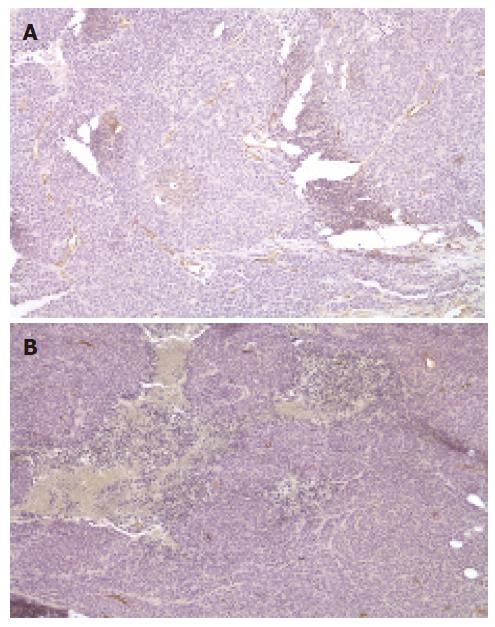
We found that delivery of KAL could significantly reduce growth of tumors, demonstrating that the treatment method was effective. Since KAL is an antiangiogenic inhibitor, in order to determine whether the suppression of tumor growth in the mice injected with rAAV-KAL was related to the antiangiogenic ability of the transgene product, the tumor blood vasculature was examined by staining for endothelial cell antigen CD34 (Figure 3). A significant reduction in microvessel density was observed in rAAV-KAL [73 ± 29 vessels/high power field (hpf), P < 0.01] compared with control mice (236 ± 67 vessels/hpf).
Tumor growth retardation could also be a result of reduction in cell proliferation. To quantitatively compare the proliferation index of tumors in different groups, tumor sections were stained for expression of Ki-67. Ki-67 is strictly expressed in proliferating cells and is commonly used as a marker for cell proliferation. Treatment with rAAV-KAL decreased the proportion of proliferating cells (Ki-67 positive cells) in tumors compared with the control group. Based on the counting of 10 randomly selected microscopic fields, the proliferation index was significantly decreased from 63% ± 9% in the control group to 41 ± 6 % in the rAAV-KAL group (P < 0.01).
Our present data showed that the application of the angiogenic inhibitor KAL suppressed angiogenesis and resulted in growth retardation of colon tumors. CD34 staining of the HCT-116 tumors revealed a significant direct correlation between MVD in histological sections of cancer and size of the tumor. This finding demonstrated that continuous release of KAL in mice could successfully decrease the MVD in HCT tumors, thereby blocking angiogenesis effectively. Ki-67 protein is widely known as an appropriate and useful marker of the proliferating fraction within a given cell population. Since Ki-67 expression provides information on the proliferating status of the tumor cells, it should give good insight into the effect of treatment. The success of the current treatment method lays an important foundation, not just for colon tumor treatment, but also for anti-angiogenic gene therapy.
Miao et al found that human KAL significantly inhibits both VEGF and bFGF induced proliferation, migration, and adhesion of primary cultured human endothelial cells in vitro, and attenuates both VEGF and bFGF induced increases in capillary density and hemoglobin content in subcutaneously implanted Matrigel plugs in vivo[6]. KAL is also a heparin binding protein. The major heparin-binding domain was identified in the region between the H helix and C2 sheet of KAL, which contains clusters of positively charged residues. KAL may act by competing with VEGF and bFGF binding to heparan-sulfate proteoglycans, a low affinity-binding site, and thus suppressing VEGF- and bFGF-binding activity and the angiogenesis signaling cascades induced by VEGF and bFGF.
As a broad-spectrum angiogenesis inhibitor, KAL inhibits angiogenesis mediated by its heparin-binding activity, which is similar to that of endostatin[13]. It has become clear that various growth factors and lymphokines are required to bind to two distinct classes of cell surface receptors to elicit a signal[14]. In many ligand-receptor systems, ligands bind first to an abundant low-affinity receptor, which draws the ligand onto the cell surface and then links it to a second, high-affinity receptor that transduces the signal into cells. In addition, KAL is a specific serine proteinase inhibitor (serpin) for human tissue kallikrein. Like plasmin, tissue kallikrein may have a role in degrading extracellular matrix to promote tumor invasion. Our study results confirmed KAL’s multifunction purpose for tumor inhibition.
With the role of angiogenesis in tumor growth and progression firmly established, considerable efforts have been directed to antiangiogenic therapy as a new modality to treat human cancers. There is much enthusiasm for the role that antiangiogenic agents may play in preventive therapy. Nevertheless, it is still unclear whether KAL can regress a tumor completely, even after prolonged treatment. Many leaders in the field of angiogenesis now believe that some of the most important future cancer therapies may not completely eradicate all tumor cells in an individual, but instead, may turn cancer into a chronic manageable disease[5]. Gene therapy strategies leading to increased production of endogenous angiogenesis inhibitors would seem perfectly suited to support such an approach by tipping the balance toward a more antiangiogenic state.
Antiangiogenic approaches should be greatly encouraged, since the FDA has recently approved the angiogenesis inhibitor avastin and the SFDA approved endostatin. Because of the difficulties and high costs of manufacturing numerous endogenous inhibitors of angiogenesis, and because of the need for chronic administration of these agents, gene therapy remains an exciting strategy to circumvent these difficulties.
AAV based vectors are now being used for clinical gene transfer for cystic fibrosis, hemophilia, and Canavan’s disease. Although recombinant adenoviral vectors have been utilized for a majority of both preclinical and clinical trials in cancer gene therapy, studies in animal models have suggested therapeutic benefits for tumor treatment using AAV vectors[7]. T-cell mediated cytoxicity to AAV vectors has not been observed even though AAV vectors can induce strong humoral immune responses. AAV can initiate long-term transgene expression and this transduction is attributed to episomal concatamer formation without integration into the host chromosome. Therefore, AAV vectors appear to be less mutagenic than other virus vectors. With new serotypes and the potential to develop targeting vectors, AAV holds great promise as a viral vector delivering therapeutic genes such as immune regulation and antiangiogenesis genes for cancer gene therapy.
In addition to AAV studies, the understanding of tumor development at the biological and molecular biological level will lead to the discovery of strong, efficient, and specific enhancers/promoters in tumor cells. Utilization of regulatory systems will avoid the undesired side effect of systemic transgene expression delivered by AAV vectors for immune-modulation and antiangiogenesis. As better vectors are developed, combination strategies continue to evolve, and there is increased understanding of the complex role that endogenous angiogenesis inhibitors play in tumor growth. Antiangiogenic gene therapy will certainly be evaluated in future clinical trials.
Colon cancer is one of the most common cancers in the world, with a high propensity to metastasize. Surgical resection currently remains the only curative treatment for colon cancer. Since the majority of deaths with colon cancer result from metastatic disease, inhibition of growth and metastasis of colon cancer is expected to become an effective treatment.
Antiangiogenesis strategies have been increasing and have been proven to be an attractive strategy for colon cancer therapy, as they are less toxic than conventional chemotherapy and they have a lower risk of drug resistance. Antiangiogenesis strategies can also transiently ‘normalize’ structure and function of tumor vasculature to make it more efficient for drug delivery and increase the efficacy of conventional therapies. Encouragingly, recent studies have demonstrated it is feasible to complete inhibition of neovascular growth in tumors by attacking multi-angiogenesis mechanisms.
There is growing evidence linking KAL to a role in the inhibition of angiogenesis. In contrast to previous reports that antiangiogenic inhibitors inhibited endothelial cells only, the results of this study clearly showed that KAL not only significantly inhibited VEGF and bFGF induced proliferation, migration, and adhesion of endothelial cells, but also suppressed the proliferation of tumor cells. The multi characteristics of KAL suggest that it is a promising candidate for a colon tumor angiogenesis inhibitor.
rAAV-mediated expression of KAL inhibits the growth of xenograft colon cancer by 78% compared with controls. Lack of toxicity may favor the clinical use of rAAV-KAL, thus demonstrating its potential in a range of clinical applications of therapy. Furthermore, rAAV-KAL may provide an effective form of therapy for other cancers in future. Elucidating the suppression of proliferation of tumor cells by KAL will provide a better understanding of the mechanism of cancer therapy.
Tumor angiogenesis: the proliferation of a network of blood vessels that penetrates into cancerous growths, supplying nutrients and oxygen and removing waste products. Tumor angiogenesis actually starts with cancerous tumor cells releasing molecules that send signals to surrounding normal host tissue.
This paper provides sufficient and new data of KAL’s unique advantage for colon tumor treatment, and that a KAL based gene therapy has great potential therapeutic value.
S- Editor Liu Y L- Editor Knapp E E- Editor Wang HF
| 1. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7733] [Article Influence: 368.2] [Reference Citation Analysis (1)] |
| 2. | Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 792] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 3. | Kisker O, Becker CM, Prox D, Fannon M, D'Amato R, Flynn E, Fogler WE, Sim BK, Allred EN, Pirie-Shepherd SR. Continuous administration of endostatin by intraperitoneally implanted osmotic pump improves the efficacy and potency of therapy in a mouse xenograft tumor model. Cancer Res. 2001;61:7669-7674. [PubMed] |
| 4. | Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2432] [Cited by in RCA: 2466] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 5. | Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427:787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 362] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 6. | Miao RQ, Agata J, Chao L, Chao J. Kallistatin is a new inhibitor of angiogenesis and tumor growth. Blood. 2002;100:3245-3252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Li C, Bowles DE, van Dyke T, Samulski RJ. Adeno-associated virus vectors: potential applications for cancer gene therapy. Cancer Gene Ther. 2005;12:913-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Xu R, Sun X, Tse LY, Li H, Chan PC, Xu S, Xiao W, Kung HF, Krissansen GW, Fan ST. Long-term expression of angiostatin suppresses metastatic liver cancer in mice. Hepatology. 2003;37:1451-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Sun X, Krissansen GW, Fung PW, Xu S, Shi J, Man K, Fan ST, Xu R. Anti-angiogenic therapy subsequent to adeno-associated-virus-mediated immunotherapy eradicates lymphomas that disseminate to the liver. Int J Cancer. 2005;113:670-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Xu R, Janson CG, Mastakov M, Lawlor P, Young D, Mouravlev A, Fitzsimons H, Choi KL, Ma H, Dragunow M. Quantitative comparison of expression with adeno-associated virus (AAV-2) brain-specific gene cassettes. Gene Ther. 2001;8:1323-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 143] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Shi J, Zheng D, Liu Y, Sham MH, Tam P, Farzaneh F, Xu R. Overexpression of soluble TRAIL induces apoptosis in human lung adenocarcinoma and inhibits growth of tumor xenografts in nude mice. Cancer Res. 2005;65:1687-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4014] [Cited by in RCA: 4088] [Article Influence: 120.2] [Reference Citation Analysis (0)] |
| 13. | Sasaki T, Larsson H, Kreuger J, Salmivirta M, Claesson-Welsh L, Lindahl U, Hohenester E, Timpl R. Structural basis and potential role of heparin/heparan sulfate binding to the angiogenesis inhibitor endostatin. EMBO J. 1999;18:6240-6248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 163] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Gallagher JT. Heparan sulfate: growth control with a restricted sequence menu. J Clin Invest. 2001;108:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 238] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 15. | Diao Y, Xu RA, Wang GJ, Zhao XC. Adeno-associated virus mediated expression of human erythropoietin in vitro. Acta Pharmacol Sin. 2002;23:55-58. [PubMed] |
| 16. | Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1165] [Cited by in RCA: 1080] [Article Influence: 47.0] [Reference Citation Analysis (0)] |