Published online Sep 14, 2007. doi: 10.3748/wjg.v13.i34.4589
Revised: June 2, 2007
Accepted: June 9, 2007
Published online: September 14, 2007
AIM: To investigate the safety and outcome of gastrectomy for patients with gastric cancer and non-uremic renal failure (NURF).
METHODS: One hundred forty-seven patients who underwent gastrectomy for carcinoma were retrospectively divided into two groups: a group with Ccr values of ≥ 50 mL/min (Group 1; n = 110), and one with Ccr values of ≥ 20 to < 50 mL/min (Group 2; n = 37). Preoperative patient characteristics, intraoperative parameters (including operation time and blood loss), and postoperative management and complications were evaluated.
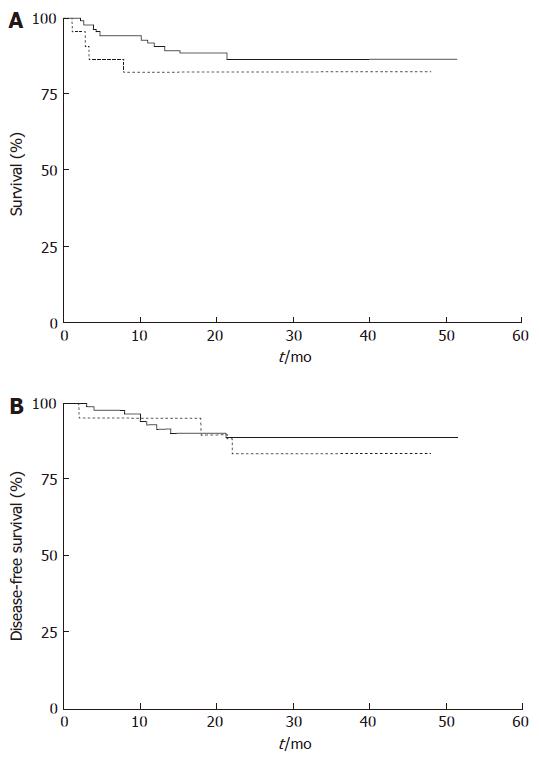
RESULTS: There were no statistically significant differences between the two groups in operation time (297.9 min vs 272.6 min, P = 0.137) or blood loss (435 mL vs 428 mL, P = 0.078). The differences in postoperative complications and hospital stay between the groups were not statistically significant. None of the patients in Group 2 required dialysis therapy, and no patients died due to gastrectomy or gastrectomy-related causes. The overall 4-year survival rates in Groups 1 and 2 were 86.6% and 81.8%, respectively (P = 0.48), and the corresponding 4-year disease-free survival rates for stageI, II, and III patients were 88.7% and 83.5%, respectively (P = 0.65).
CONCLUSION: Gastrectomy can be performed as safely in patients with NURF similar to patients with normal renal function.
- Citation: Mori S, Sawada T, Hamada K, Kita J, Shimoda M, Tagaya N, Kubota K. Gastrectomy for patients with gastric cancer and non-uremic renal failure. World J Gastroenterol 2007; 13(34): 4589-4592
- URL: https://www.wjgnet.com/1007-9327/full/v13/i34/4589.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i34.4589
Gastric cancer is the most common cancer in Japan, and the prognosis of affected patients has been improving[1,2]. The surgical procedure for gastric cancer consists of gastrectomy, with regional and extended lymph node dissection. Although advances in surgical techniques and management have made it possible to perform gastrectomy safely, renal dysfunction remains a major risk factor for perioperative management.
Renal dysfunction is classified into non-uremic and uremic stages. Patients with non-uremic renal failure (NURF) are defined as having impaired renal function but are dependent on their own kidneys. Patients with NURF require special attention to prevent deterioration of renal function and avoid the need for dialysis therapy when undergoing gastrectomy. Recently, due to the increase of the aged population and the incidence of diabetes mellitus, patients with gastric cancer associated with NURF have been increasing[3]. However, there have been no published reports focusing on gastrectomy in such patients. In the present study, we retrospectively evaluated the results of pre-, intra-, and postoperative management and the outcome of gastrectomy in patients with gastric cancer associated with NURF to assess the safety of gastrectomy in such patients.
A total of 147 patients who underwent gastrectomy for carcinoma between January 2003 and December 2005 were included in this study. In all patients, renal dysfunction was evaluated by the creatinine clearance test. NURF was defined on the basis of a creatinine clearance rate (Ccr) of > 20 to < 50 mL/min according to the New York Heart Association criteria[4,5].
The patients were divided into two groups based on the Ccr value: a group with a Ccr of > 50 mL/min (Group 1; normal renal function: n = 110) and a group with a Ccr of > 20 to < 50 mL/min (Group 2; NURF: n = 37). The characteristics of the patients are shown in Table 1.
| G1 | G2 | P-value | |
| Number | 110 | 37 | |
| Sex (male/female) | 80/30 | 27/10 | P = 0.98 (χ2) |
| Age | 66.6 ± 9.4 | 73.2 ± 9.4 | P < 0.001 |
| BMI | 22.2 ± 3.3 | 21.5 ± 3.6 | P = 0.24 |
| BUN (mg/dL) | 13.5 ± 4.0 | 17.0 ± 4.6 | P < 0.001 |
| Cr (mg/dL) | 0.7 ± 0.2 | 0.9 ± 0.2 | P < 0.001 |
| Ccr (mL/min) | 82.5 ± 15.9 | 38.1 ± 8.8 | P < 0.001 |
Our treatment strategy for gastric carcinoma in patients with NURF is to perform gastrectomy with regional lymph node dissection to the same extent as that for patients with normal renal function. We do not reduce the extent of lymph node dissection in patients with NURF. If patients have metastases in organs such as the liver and lung (Stage IV), removal of gastric cancer by distal gastrectomy or total gastrectomy without regional lymph node dissection is performed.
Our management strategy for patients with NURF is to maintain intraoperative and postoperative urine volume at more than 1 mL/kg per hour. To obtain this target, adequate fluid infusion and administration of diuretics are employed.
For adjuvant chemotherapy, we use oral administration of fluorouracil, and for patients whose clinical stage is more advanced than stage III, an intravenous infusion of the anti-neoplastic agents fluorouracil and cisplatin is given.
Patients’ preoperative characteristics including serum blood nitrogen urea (BUN) and creatinine (Cr), surgical methods, TNM classification, surgery-related data such as operation time, blood loss, and postoperative complications, were compared between Groups 1 and 2. Postoperative increase of BUN and Cr was calculated by dividing the maximum postoperative value by the preoperative value, and multiplying by 100.
The data are expressed as mean ± SD or median value (minimum-maximum). Statistical analyses for means and medians were performed by Mann-Whitney U test. Statistical analyses of surgical procedures, lymph node dissection, and TNM classification were performed using the chi-squared test. The 4-year survival and the 4-year disease-free survival rates were analyzed by the Kaplan-Meier method, and statistical analysis was performed by log-rank test. For the calculation of 4-year disease-free survival, only patients whose clinical stages wereI, II, and III in Groups 1 (n = 93) and 2 (n = 28) were included. Differences at P < 0.05 were considered to be significant.
Preoperative clinical data are shown in Table 1. The mean patient age was higher in Group 2 (73.2 ± 9.4 years) than in Group 1 (66.6 ± 9.4 years). Higher Cr and BUN values were observed in Group 2 than in Group 1, and the differences were statistically significant. The Ccr value was significantly lower in Group 2 (38.1 ± 8.8 mL/min) than in Group 1 (82.5 ± 15.9 mL/min).
Table 2 and Table 3 shows details of the surgical procedure and the extent of lymph node dissection. There were 6 cases of remnant gastrectomy in Group 1 and no such cases in Group 2. Also there was one case of partial gastrectomy in Group 2 and no such case in Group 1. However, there were no significant differences in the surgical procedure between the two groups.
| G1 | G2 | P-value | |
| Distal gastrectomy | 60 (54.5) | 20 (54.1) | 0.96 |
| Total gastrectomy | 44 (40) | 15 (40.5) | 0.95 |
| Proximal gastrectomy | 0 | 1 (2.7) | 0.08 |
| Remnant gastrectomy | 6 (5.5) | 0 | 0.33 |
| Partial gastrectomy | 0 | 1 (2.7) | 0.08 |
| G1 | G2 | P-value | ||
| Distal gastrectomy | D0 | 53.30 | 45.00 | 0.24 |
| D1 | 38.30 | 25.00 | 0.036 | |
| D2 | 8.30 | 30.00 | < 0.001 | |
| Total gastrectomy | D0 | 25.00 | 26.70 | 0.78 |
| D1 | 68.20 | 40.00 | < 0.001 | |
| D2 | 6.80 | 33.30 | < 0.001 |
The details of the TNM classification are shown in Table 4. The incidence of advanced gastric cancer exceeding stage II was significantly higher in Group 2 than in Group 1 (Group 1; 40.9%, Group 2; 59.5%, P < 0.05).
| G1 | G2 | |
| Stage IA | 45 (40.9) | 7 (18.9) |
| IB | 20 (18.2) | 8 (21.6) |
| Stage II | 10 (9.1) | 5 (13.5) |
| Stage IIIA | 13 (11.8) | 7 (18.9) |
| IIIB | 5 (4.5) | 1 (2.7) |
| Stage IV | 17 (15.5) | 9 (24.3) |
Table 5 shows the operative data. Operation times in Groups 1 and 2 were 279 (139-643) min and 259 (130-521) min, respectively (P = 0.14), and operative blood losses were 324 (26-2314) mL and 250 (42-3251) mL, respectively (P = 0.048). There were no significant inter-group differences in intraoperative urine volume, occurrence of postoperative complications, or median postoperative hospital stay.
| G1 | G2 | P-value | |
| Operation time (min) | 279 (139-643) | 259 (130-521) | 0.14 |
| Operative blood loss (mL) | 324 (26-2314) | 250 (42-3251) | 0.0481 |
| Operative urine volume (mL/kg per hour) | 0.92 (0.25-7.31) | 0.71 (0.17-3.03) | 0.17 |
| Postoperative complications (%) | 18.2 | 16.2 | 0.79 |
| Postoperative hospital stay (d) | 15 (11-73) | 15 (11-80) | 0.78 |
Postoperative percentage increases in BUN and Cr are shown in Table 6. There were no significant increases in BUN and Cr after surgery in either group. Furthermore, none of the patients in either group were placed on hemodialysis after gastrectomy.
| G1 | G2 | P-value | |
| BUN | 19.5% (-55.6-180) | 11.5% (-47.6-166.7) | P > 0.05 |
| Cr | 9.0% (-20.5-700) | 0.0% (-20-42.9) | P > 0.05 |
Details of postoperative complications are shown in Table 7. The incidences of these complications did not differ between the two groups. No patients died as a result of the gastrectomy procedure or of gastrectomy-related causes.
| G1 | G2 | |
| Pneumonia | 6 | 3 |
| Pancreatitis | 4 | 0 |
| Anastomotic leakage | 4 | 1 |
| Drain infection | 3 | 0 |
| Catheter infection | 2 | 1 |
| Ileus | 1 | 0 |
| Others | 3 | 3 |
The 4-year survival rates in Groups 1 and 2 were 86.6% and 81.8%, respectively (P = 0.48) (Figure 1A), and the 4-year disease-free survival rates were 88.7% and 83.5%, respectively (P = 0.65) (Figure 1B).
The critical factor to consider when performing curative gastrectomy for patients with NURF is to perform the procedure safely to prevent deterioration of renal function and avoid the need for dialysis therapy. Patients with NURF have several risk factors for major surgery. It is known that renal dysfunction is frequently associated with cardiac dysfunction, a condition known as a cardio-renal syndrome[6]. In cardio-renal syndrome, activation of the renin-angiotensin-aldosterone system, imbalance of reactive oxygen species, and irritation of the sympathetic nervous system result in acceleration of coronary sclerosis, cardiac hypertrophy, impairment of cardiac microcirculation, and hypertension[7]. It is also known that renal anemia is a solid risk factor for cardiovascular dysfunction (cardio-renal anemia syndrome)[8]. Therefore, when performing major surgery in patients with NURF, careful evaluation of the cardiovascular system is essential.
Patients with NURF have impaired blood-coagulation function[9]. Increased perioperative bleeding is the main cause for the deterioration of perioperative renal function, and often results in other postoperative complications. However, in the present study, intraoperative blood loss was significantly higher in Group 1 than in Group 2, regardless of the fact that there were no significant differences in the surgical procedures, and the more extensive lymph node dissection in Group 2 than in Group 1 (Table 2 Table 3). Proper intraoperative hemostasis makes it possible to perform gastrectomy safely for patients with NURF.
There was no significant difference in the postoperative morbidity rates between the two groups. Postoperative complications included pneumonia, pancreatitis, anastomotic leakage, drain infection, catheter infection, ileus, and others, but none of these complications are unique to patients with NURF. The median postoperative hospital stay was 15 d in both groups, and there was no statistically significant difference between them.
Patients with renal failure are immunocompromised, and the occurrence of perioperative infection is higher than in the normal population[10]. However, in this study, there was no significant difference in postoperative infection between the two groups. We routinely use cephalosporin-antibiotics for 3 d after surgery, and the dose of antibiotics was the same in both groups, no cases of drug-induced renal function deterioration being observed in either of them. In terms of the TNM classification, the frequency of advanced gastric cancer above stage II was significantly higher in Group 2 than in Group 1 (Table 4). The immunocompromised state of patients with NURF may be the reason why more advanced-stage gastric cancers were present in Group 2 than in Group 1.
Previous reports have suggested that impaired innate and acquired immunity in patients with end-stage renal disease increases the incidence of cancer, the rate of recurrence after surgery, and decreases the survival rate[11-14]. In one study, relative risks of gastric cancer in patients with end-stage renal disease in Australia, New Zealand and America were 1.2[15]. In our study, the survival and disease-free survival rates for gastric cancer after curative surgery in patients with NURF were the same as those in patients with normal renal function. Thus, removal of gastric cancer with a sufficient normal stomach, associated with extended lymph node dissection, enables patients with NURF to achieve a satisfactory outcome.
In conclusion, with accurate preoperative evaluations, appropriate operative procedures and perioperative management, gastrectomy for carcinoma including extended lymph node dissection in patients with NURF can be performed as safely as in patients with normal renal function.
Renal dysfunction is classified into non-uremic and uremic stages. Patients with non-uremic renal failure (NURF) are defined as having impaired renal function but dependent on their own kidneys. Crucial issue in the surgery for the patients with NURF is to prevent deterioration of renal function and avoid the need for dialysis.
We retrospectively evaluated the results of pre-, intra-, and postoperative management and the outcome of gatrectomy in patients with gastric cancer associated with NURF to assess the safety of gastrectomy in such patients.
With accurate preoperative evaluations, appropriate operative procedures and perioperative management, gastrectomy for carcinoma including extended lymph node dissection in patients with NURF can be performed as safely as in patients with normal renal function.
Malignant diseases occur likely in patients with NURF and within 2 years of the introduction of hemodialysis. Findings of the present study fascillitate the aggressive surgery in such patients.
Non-uremic renal failure (NURF): patients having impaired renal function but dependent on their own kidneys.
This is an unique study and as such worthy of acceptance. It is well written and presented. I think it would benefit if the authors made more of their management in maintaining urinary output both during and after the operation.
S- Editor Liu Y L- Editor Alpini GD E- Editor Lu W
| 1. | Ajiki W, Kinoshita N, Tsukuma H, Oshima A. Cancer incidence and incidence rates in Japan in 1996: estimates based on data from 10 population-based cancer registries. Jpn J Clin Oncol. 2001;31:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Saka M, Sasako M. [Informed consent for gastric cancer surgery]. Nihon Geka Gakkai Zasshi. 2007;108:10-14. [PubMed] |
| 3. | Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Modern surgery for gastric cancer--Japanese perspective. Scand J Surg. 2006;95:232-235. [PubMed] |
| 4. | Oken DE. Criteria for the evaluation of the severity of established renal disease. Nephron. 1970;7:385-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Winearls CG. Clinical evaluation and manifestations of chronic renal failure. In: Johnson R, Feehally J, editors. Comprehensive Clinical Nephrology. 1st ed. London: Mosby; 68.1. . |
| 6. | Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7995] [Cited by in RCA: 8530] [Article Influence: 406.2] [Reference Citation Analysis (0)] |
| 7. | Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. The severe cardiorenal syndrome: 'Guyton revisited'. Eur Heart J. 2005;26:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 297] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 8. | Jurkovitz CT, Abramson JL, Vaccarino LV, Weintraub WS, McClellan WM. Association of high serum creatinine and anemia increases the risk of coronary events: results from the prospective community-based atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2003;14:2919-2925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Weintraub SL, Wang YZ, Hunt JP, O'Leary JP. Principles of preoperative and operative surgery. Textbook of Surgery. 17th ed. Philadelphia, Pennsylvania: Elsevier Saunders 2004; 221. |
| 10. | Newberry WM, Sanford JP. Defective cellular immunity in renal failure: depression of reactivity of lymphocytes to phytohemagglutinin by renal failure serum. J Clin Invest. 1971;50:1262-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 151] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Inamoto H, Ozaki R, Matsuzaki T, Wakui M, Saruta T, Osawa A. Incidence and mortality patterns of malignancy and factors affecting the risk of malignancy in dialysis patients. Nephron. 1991;59:611-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Iseki K, Osawa A, Fukiyama K. Evidence for increased cancer deaths in chronic dialysis patients. Am J Kidney Dis. 1993;22:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Cucković C, Djukanović L, Janković S, Stanojcić A, Dragićević P, Radmilović A, Lambić L, Stojánović M, Milić M, Baković J. Malignant tumors in hemodialysis patients. Nephron. 1996;73:710-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Sawada T, Kita J, Rokkaku K, Kato M, Shimoda M, Kubota K. Hepatectomy in patients with nonuremic minimal renal failure. J Gastrointest Surg. 2006;10:740-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, Wolfe RA, Jones E, Disney AP, Briggs D. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet. 1999;354:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 609] [Article Influence: 23.4] [Reference Citation Analysis (0)] |