Published online Sep 14, 2007. doi: 10.3748/wjg.v13.i34.4566
Revised: June 20, 2007
Accepted: June 23, 2007
Published online: September 14, 2007
AIM: To establish an ideal model of multiple organ injury of rats with severe acute pancreatitis (SAP).
METHODS: SAP models were induced by retrograde injection of 0.1 mL/100 g 3.5% sodium taurocholate into the biliopancreatic duct of Sprague-Dawley rats. The plasma and samples of multiple organ tissues of rats were collected at 3, 6 and 12 h after modeling. The ascites volume, ascites/body weight ratio, and contents of amylase, endotoxin, endothelin-1 (ET-1), nitrogen monoxidum (NO), phospholipase A2 (PLA2), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) in plasma were determined. The histological changes of multiple organs were observed under light microscope.
RESULTS: The ascites volume, ascites/body weight ratio, and contents of various inflammatory mediators in blood were higher in the model group than in the sham operation group at all time points [2.38 (1.10), 2.58 (0.70), 2.54 (0.71) vs 0.20 (0.04), 0.30 (0.30), 0.22 (0.10) at 3, 6 and 12 h in ascites/body weight ratio; 1582 (284), 1769 (362), 1618 (302) (U/L) vs 5303 (1373), 6276 (1029), 7538 (2934) (U/L) at 3, 6 and 12 h in Amylase; 0.016 (0.005), 0.016 (0.010), 0.014 (0.015) (EU/mL) vs 0.053 (0.029), 0.059 (0.037), 0.060 (0.022) (EU/mL) at 3, 6 and 12 h in Endotoxin; 3.900 (3.200), 4.000 (1.700), 5.300 (3.000) (ng/L) vs 41.438 (37.721), 92.151 (23.119), 65.016 (26.806) (ng/L) at 3, 6 and 12 h in TNF-α, all P < 0.01]. Visible congestion, edema and lamellar necrosis and massive leukocytic infiltration were found in the pancreas of rats of model group. There were also pathological changes of lung, liver, kidney, ileum, lymphonode, thymus, myocardium and brain.
CONCLUSION: This rat model features reliability, convenience and a high achievement ratio. Complicated with multiple organ injury, it is an ideal animal model of SAP.
- Citation: Zhang XP, Ye Q, Jiang XG, Ma ML, Zhu FB, Zhang RP, Cheng QH. Preparation method of an ideal model of multiple organ injury of rat with severe acute pancreatitis. World J Gastroenterol 2007; 13(34): 4566-4573
- URL: https://www.wjgnet.com/1007-9327/full/v13/i34/4566.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i34.4566
Severe acute pancreatitis (SAP) is an acute clinical disease, featuring acute onset, rapid progression, high incidence of complications and high mortality. Its pathogenesis has still not been fully elucidated till now. There is also no ideal therapy. It is quite worthwhile to rely on a SAP animal model to strengthen the study of pathogenesis and treatment of SAP. There are many requirements on an ideal SAP experimental animal model. There should be simple and reliable methods, and a low cost and high achievement ratio. The pathological changes, progression and response to treatment of the model also should be similar to those of the human body. In this study, SAP animal models were induced by retrograde injection of 3.5% sodium taurocholate into the biliopancreatic duct of Sprague-Dawley (SD) rats. The feasibility and significance of the model were studied. And the preparation methods for an ideal model of SAP rats with multiple organ injury were established.
Ninety clean grade healthy male SD rats with a body weight of 250-300 g were purchased from the Experimental Animal Center of Zhejiang Medical School.
Sodium taurocholate and sodium pentobarbital purchased from USA Sigma Company. Dexamethasone injection purchased from Zhejiang Xinchang Pharmaceutical Company. The full automatic biochemical analyzer was used to determine the plasma amylase level (U/L). Plasma endotoxin tachypleus amebocyte lysate kit was purchased from Shanghai Yihua Medical Science and Technology Corporation (Institute of Medical Analysis in Shanghai), the calculation unit for content is EU/mL. The serum nitrogen monoxidum (NO) kit was purchased from Nanjing Jiancheng Bioengineering Research Institute, the calculation units for content is μmol/L. TNF-α ELISA kit was purchased from Jingmei Bioengineering Corporation, the calculation unit for content is pg/mL (ng/L). The serum secretory phospholipase A2 enzyme Assay ELA kit (phospholipase A2) was purchased from R&D system Ins and the calculation unit for content is U/mL. The serum Endothelin-1 ELA kit (Endothelin-1) was purchased from Cayman chemical company (Catalog Number: 583 151) and the calculation unit for content is ng/L (pg/mL). The above determinations were all operated according to the instructions of the kits.
The experimental rats were randomly assigned to the model group and control group (45 rats/group). The Aho’s method was adopted and the SAP rats of model group were prepared by retrograde injection of 3.5% sodium taurocholate into biliopancreatic duct through segmental eqidural catheter via duodenal papilla. Another 45 rats were assigned to the sham operation group where only exploratory laparotomy was performed. The above-mentioned groups were then divided into 3, 6 and 12 h group, with 15 rats in each group. The following steps were performed at 3, 6 and 12 h after operation: (1) Rat mortality of all groups was observed. Then the rats were sacrificed in batches. The ascites/body weight ratio was observed. (2) The samples of pancreas, lung, liver, kidney, terminal ileum, ileocecal junction lymphonode, thymus, myocardium and brain were collected and their pathological changes were observed. (3) After collecting blood from the heart, the ascites volume, ascites/body weight ratio and contents of amylase and endotoxin in plasma and contents of endothelin-1 (ET-1), nitrogen monoxidum (NO), phospholipase A2 (PLA2), tumor necrosis factor-α (TNF-α) and IL-6 in serum were determined.
Fasting with water restraint was imposed on all rat groups 12 h prior to the operation. The rats were anesthetized by intraperitoneal injection of 2% sodium pentobarbital (0.25 mL/100 g) after which lay and fixed the rats, and performed the routine shaving, disinfection and draping.
Model group: after entering abdomen via median epigastrium incision, confirmed the bile-pancreatic duct and hepatic hilus common hepatic duct, disclosed the pancreas, identified the duodenal papilla inside the duodenum duct wall, and then used a No. 5 needle to drill a hole in the mesenterium avascular area. After inserting a segmental eqidural catheter into the duodenum cavity via the hole, inserted into the bile-pancreatic duct toward the direction of papilla in a retrograde way, used the microvascular clamp to nip the catheter head temporarily and meanwhile used another microvascular clamp to temporarily occlude the common hepatic duct at the confluence of hepatic duct. After connecting the eqidural catheter end with the transfusion converter, transfused 3.5% sodium taurocholate 0.1 mL/100 g by retrograde transfusion via the microinjection pump at the speed of 0.2 mL/min. Stayed for 4 min after injection and removed the microvascular clamp and epidural catheter. After checking for bile leakage, sutured the hole in the duodenum lateral wall. Used the disinfected cotton ball to absorb up the anesthetic in the abdominal cavity and close the abdomen.
The statistical analysis was conducted to the arranged experimental results by applying the SPSS11.5 software. The Kruskal-Wallis test or variance analysis (only applied to PLA2) was applied to the two-group comparison. The Bonfferoni test was also applied to comparison. There are statistical significances when P≤ 0.05.
Model group: Mortality respectively was 0% (0/15), 0% (0/15) and 13.33% (2/15) at 3, 6 and 12 h. The sham operation group survived at all time points with 100% survival.
The volume was higher in model group than in sham operation group at all time points (P < 0.001) (Table 1).
| Groups | 3 h | 6 h | 12 h |
| Sham operation group | 0.50 (0.00) | 0.70 (0.50) | 0.60 (0.30) |
| Model group | 7.20 (2.00) | 6.40 (2.30) | 7.90 (1.70) |
The coefficient was higher in model group than in sham operation group at all time points (P < 0.001) (Table 2).
| Groups | 3 h | 6 h | 12 h |
| Sham operation group | 0.20 (0.04) | 0.30 (0.30) | 0.22 (0.10) |
| Model group | 2.38 (1.10) | 2.58 (0.70) | 2.54 (0.71) |
The contents were higher in model group than in sham operation group at all time points (P < 0.001) (Table 3).
| Index | Sham operation group | Model group | ||||
| 3 h | 6 h | 12 h | 3 h | 6 h | 12 h | |
| Amylase (U/L) | 1582 (284) | 1769 (362) | 1618 (302) | 5303 (1373) | 6276 (1029) | 7538 (2934) |
| Endotoxin (EU/mL) | 0.016 (0.005) | 0.016 (0.01) | 0.014 (0.015) | 0.053 (0.029) | 0.059 (0.037) | 0.060 (0.022) |
| ET-1 (ng/L) | 15.293 (4.231) | 16.275 (3.180) | 14.173 (2.556) | 24.745 (1.011) | 25.625 (7.973) | 24.725 (3.759) |
| NO (μmol/L) | 7.500 (5) | 7.500 (5) | 10.000 (5) | 65.000 (7.5) | 62.500 (38.8) | 74.100 (26.2) |
| TNF-α (ng/L) | 3.900 (3.200) | 4.000 (1.7) | 5.300 (3.000) | 41.438 (37.721) | 92.151 (23.119) | 65.016 (26.806) |
| IL-6 (ng/L) | 1.846 (0.346) | 1.743 (0.838) | 2.036 (0.818) | 5.437 (1.025) | 6.817 (0.81) | 5.356 (0.747) |
| PLA2 (U/mL) | 18.70 ± 4.40 | 16.70 ± 3.83 | 18.52 ± 11.32 | 103.69 ± 20.82 | 119.85 ± 17.74 | 121.29 ± 17.00 |
The content was higher in model group than in sham operation group at all time points (P < 0.01) (Table 3).
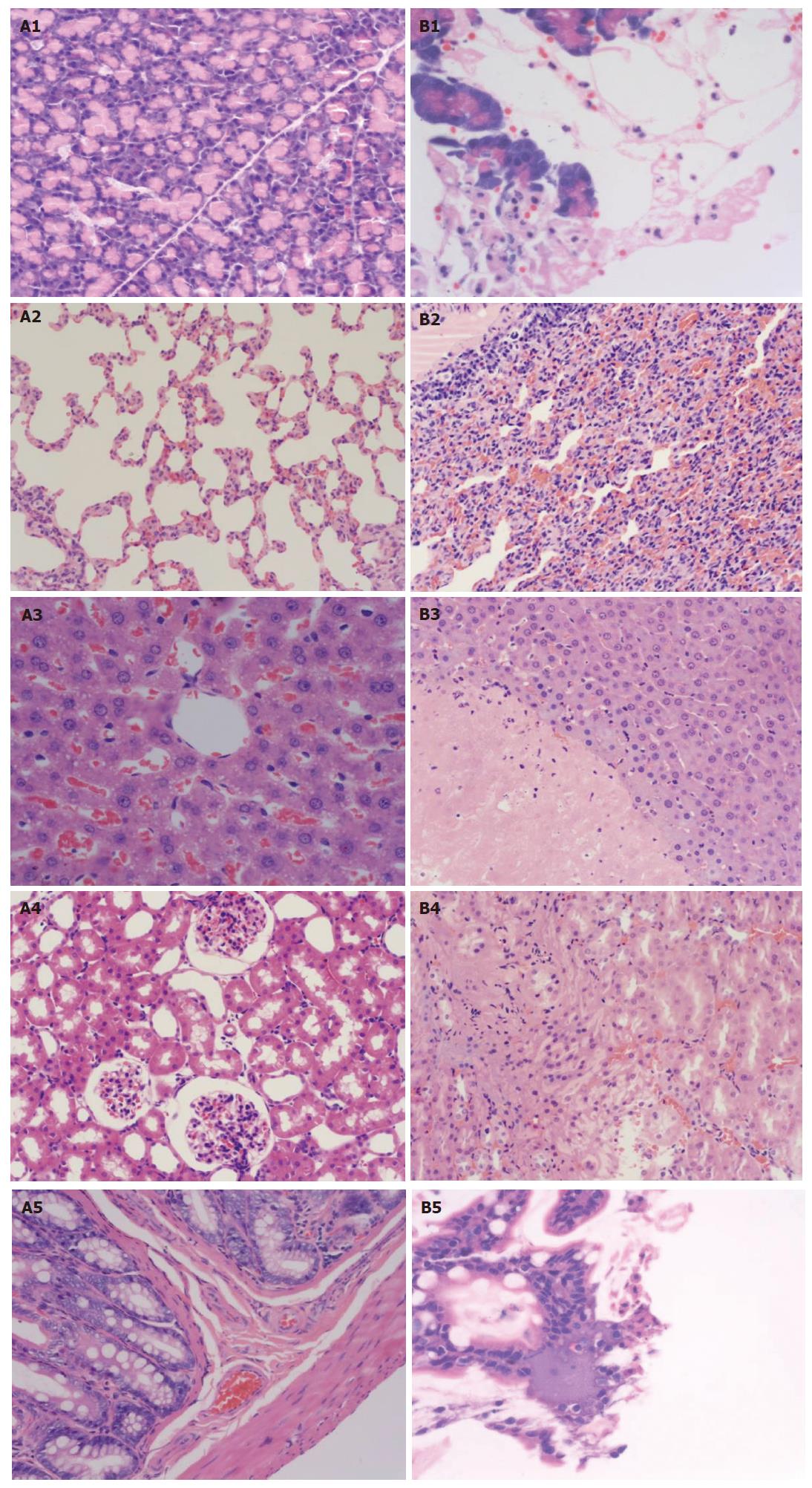
Pathological changes of pancreas: (1) Sham operation group, Gross changes: Pancreas, fat surrounding pancreas and epiploon were normal at all time points after operation. Changes under light microscope: Most cases were normal. Mild interstitial edema was found in very few cases and neutrophil infiltration was seen by chance. There was no necrosis and hemorrhage of acinar cell and fat (Figure 1 A1). (2) Model group, Gross changes: The level of gross pathological changes and changes under light microscope was higher in pancreas tail than in pancreas head. The severity of all pathological changes aggravated with time after modeling. Edema, hemorrhage and necrosis appeared in the pancreas 5 min after inducing models. There were small amounts of ascites, slightly hemorrhagic in most cases, congestion and edema of the pancreas, and hemorrhage and necrosis in some cases at 3 h. The ascites was hemorrhagic in most cases at 6 and 12 h. And the average volume was higher at 6 and 12 h than that at 3 h. The severity of edema, hemorrhage and necrosis was higher at 6 and 12 h than at 3 h, with many saponification spots in epiploon and peritoneum surrounding pancreas, gel like change and contour disappearance of pancreas, and significant hemorrhage and necrosis (Figure 1 B1). Changes under light microscope: The severity of all pathological changes aggravated with time after modeling.
There were visible interstitial congestion and edema, mild inflammatory cell infiltration, focal necrosis and mild interstitial hemorrhage in pancreas at 3 h, with lamellar necrosis and hemorrhage in some cases. There were interstitial congestion and edema, focal or lamellar necrosis and hemorrhage, more inflammatory infiltration in pancreas at 6 h. There were interstitial edema and hemorrhage, massive necrosis and hemorrhage, damaged lobule contour and massive inflammatory cell infiltration in pancreas at 12 h.
Pathological changes of lung: (1) Sham operation group: Gross changes: The color and morphous of both sides of lung were normal without bleeding point on surface or effusion in thoracic cavity. Changes under light microscope: The structure was normal in most part of lung. There were mild interstitial edema and mild inflammatory cell infiltration in a very small part. (2) Model group: Gross changes: 3 h: There were congestion and edema in pulmonary lobes of both sides, madder red bleeding points on surface of local pulmonary lobes, mild amber effusion in thoracic cavity; 6 and 12 h: There were pathological changes of both sides of lung aggravating with time after molding, prunosus plaque on lung surface, more hemorrhagic effusion in thoracic cavity. Changes under light microscope: There was edema of the lung interstitium and alveolar space, broadened alveolar wall interstitium, inflammatory cell infiltration, telangiectasis and congestion of alveolar wall, and broadened alveolar septum; 6 and 12 h: There were increased range of pathological changes of pulmonary lobes and effusion in alveolar space, edema and hemorrhage of interstitium and alveolar space, broadened alveolar septum, inflammatory cell infiltration, and lucent kytoplasm of epithelial cell in local tunica mucosa bronchiorum (Figure 1 A2 and B2).
Pathological changes of liver: (1) Sham operation group: Gross changes: The color of liver was normal without visible swelling. Changes under light microscope: There were normal hepatic tissue, mild inflammatory cell infiltration in portal area, normal morphous of hepatic cell in most cases, acidophilia denaturation in some cases, and mild dilation and congestion of sinus hepaticus in few cases (Figure 1 A3). (2) Model group: Gross changes: 3 h: There were mild liver swelling, local gray plaque in local liver of individual rats, obscure boundary; 6 and 12 h: There were pale and muddy color and congestion of the liver, irregular gray plaque or necrosis in local area. Changes under light microscope: 3 h: There were hepatic cell swelling or acidophilia denaturation, inflammatory cell infiltration in portal area, dilation and congestion in sinus hepaticus, scattered spotty necrosis in hepatic lobule; 6 h: There were visible hepatic cell swelling, increased range and area of hepatic cell necrosis, focal or massive hemorrhagic necrosis, inflammatory cell infiltration in necrotic focus, congestion in partial sinus hepaticus, bile duct proliferation in portal area and scattered necrosis of single cell (condensation and break of nucleus); 12 h: There were damaged hepatic lobule structure, further increased range and area of hepatic cell necrosis, inflammatory cell infiltration in lobule and/or portal area, congestion in sinus hepaticus (Figure 1 B3).
Pathological changes of kidney: (1) Sham operation group: Gross changes: The morphous of the kidney was normal without swelling, with no bleeding points on surface of renal cortex. Changes under light microscope: There were normal structure of renal glomerulus, tubule and interstitium in most rats, and blurry boundary of epithelial cell of renal tubule (especially proximal tubule), stegnosis and atresia of lumens, congestion of renal glomerulus and interstitial edema in few rats (Figure 1 A4).
(2) Model group: Gross changes: There was no gross change in kidney at 3 h. There were kidney swelling, tension of renal envelope, scattered bleeding points on surface of renal envelope of rats, and slightly hemorrhagic urine in pelvis in severe cases at 6 and 12 h. Changes under light microscope: 3 h: There were capillary congestion of renal glomerulus, swelling, scattered necrosis and blurry boundary of renal tubule epithelial cell, stegnosis or atresia of lumens, visible protein cast, interstitial edema and inflammatory cell infiltration. 6 and 12 h: There were capillary congestion of renal glomerulus, swelling and scattered necrosis of epithelial cell of renal tubule, interstitial edema and inflammatory cell infiltration. The floss and red cell with eosinophilic staining were found in renal glomerulus and so was homogen or red cell cast with eosinophilic staining in renal tubule. There were lumens dilation of medulla renal tubule and atrophia of epithelial cell. The pathological changes aggravated with time. There was lamellar necrosis of epithelial cell of renal tubule in few rats (Figure 1 B4).
Pathological changes of ileum: (1) Sham operation group, Gross changes: There was no intestinal dilation or congestion or edema of intestinal wall. The surface of intestinal mucosa was smooth without pathological changes such as hemorrhage and ulcer. Changes under light microscope: The structure of epithelium and microvillus of intestinal mucosa was integrated without exfoliation or necrosis. Edema of proper layer, submucous layer and placenta percreta was found in few cases. Focal necrosis of mucosa and inflammatory cell infiltration of proper layer, submucous layer and placenta percreta were found in very few cases (Figure 1 A5). (2) Model group, Gross changes: There was no intestinal change at 3 h. Visible intestinal dilation was found at 6 and 12 h with retention of certain amount of gas and liquid, congestion and edema of intestinal wall and bleeding points on surface of intestinal mucosa. Small focus of mucosal ulcer was found in severe cases. Changes under light microscope: Focal necrosis of ileum mucosa and inflammatory cell infiltration in various mucosa layers were found in most rats. There were visible congestion edema of proper layer of intestinal mucosa, dilation of central chyle vessel, exuviations of microvillus top of epithelium of intestinal mucosa and disordered structure arrangement at 6 and 12 h (Figure 1 B5).
Pathological changes of lymphonode: The morphous and structure of lymphonode were normal in sham operation group and swelled in model group with lymphonode swelling, dilation of follicle germinal center of lymphonode and lymphatic sinus, sinus cell hyperplasia, and frequently spotty necrosis in germinal center of folliculus lymphaticus. Infiltration of neutrophil and plasmocyte was found only in few cases.
Pathological changes of thymus: (1) Sham operation group: The histological findings of thymus at 3, 6 and 12 h were consistent. There were normal structure of thymus, clear boundary between cortex and medulla, about 2-1:1 of thickness ratio of cortex and medulla, visible lobule, integrated envelope, “starry sky” changes in few epithelial cells of cortex, and condensed dark purple blue nucleus. There were more epithelial reticular cell slightly stained in medulla, in star shape and with many prominences, large nucleus and rich kytoplasm. The structure was looser in medulla than in cortex. Slightly stained nucleus of epithelial cell was found only in few cases and epithelial cell was “vacuole” in individual cases. (2) Model group: The cortex was gradually thinned and expanded compared with medulla at 3, 6 and 12 h. There were “starry sky” epithelial cell, nuclear fragmentation and reduced amount of lymphocytes. The nucleus of epithelial cell in medulla was slightly stained. There were more “vacuole” epithelial cells in model group than in normal group.
Pathological changes of myocardium: (1) Gross changes: Normal manifestation and no visible change of appearance in all groups. (2) Changes under light microscope: The transverse striation of cardiac muscle fiber was clear in all cases. There was no abnormal cardiac muscle fiber in sham operation group. The fiber was normal in most cases of model group. But one case of blurry transverse striation of cardiac muscle fiber occurred at 3 h in model group. Granulation or lyses of muscle plasma of cardiac muscle fiber was found in 2, 3, 5 rats respectively at 3, 6 and 12 h. Mild inflammatory cell infiltration of myocardium interstitium and epicardium was found in one case of each group respectively at 3 h.
Pathological changes of brain: (1) Gross changes: Normal manifestation and no visible change of appearance in all groups. (2) Changes under light microscope: The pathological changes were almost invisible. The mild swelling of neuron was found in 2, 2, 3 rats of model group at 3, 6 and 12 h. Other rats were normal.
At present, preparation of SAP animal model is still the main method for study of SAP pathogenesis and evaluation of therapeutic effects of drugs. Therefore, many researchers have designed and carried out a great deal of animal experiment studies. The most commonly used living SAP animals are induced by puncture injection of biliopancreatic duct[1-4], injection under capsula pancreatic[5], ligation of pancreatic duct[6], loop close of duodenum[7], biliopancreatic duct injection in combination with intravenous infusion[8] and intraperitoneal injection[9-11]. In addition, the drugs most frequently used to induce SAP model are sodium taurocholate[12,13] that is most popular, glycodeoxycholic acid, bombesin combined with lipopolysaccharide[14,15], and L-arginine[16-18]. The rat is most frequently used. Although the above methods and drugs can successfully induce SAP model, they cannot sufficiently control the severity of pathological changes of pancreas and injury of non-pancreas organs. Therefore, it is difficult for them to accommodate the therapeutic effects and mechanism of drugs evaluated. In addition, the experimental result also will be influenced by the inconsistency of severity and range of pathological changes in pancreas and multiple non-pancreas organs in SAP models induced by the same method. Sometimes a completely opposite conclusion will be obtained. Therefore, it is necessary to establish a reliable and stable SAP model with high achievement ratio that should correctly reflect the pathological changes in pancreas and multiple non-pancreas organs during SAP and aid the evaluation of therapeutic effects.
Aho et al[1] first reported the SAP rat induction by retrograde injection of 5% sodium taurocholate through puncture of biliopancreatic duct in 1980. The method is a classic model preparation method extensively applied[19-21]. The principle of its preparation is based on the theory of bile reflux. The rat SAP will be induced directly by injuring pancreatic tissue or activating endogenous pancreatin after retrograde injection of sodium taurocholate into biliopancreatic duct. Compared with other models, this method can induce the pathological changes including blood supply disturbance of microcirculation, edema, hemorrhage and necrosis of pancreatic tissue, quite similar to the pathological features of human bile reflux pancreatitis. However, it was found in our experiment that this method could cause serious surgical trauma, blood loss and high mortality. There were also problems including unsatisfactory model induction and insensitivity to medicine intervention due to biliary fistula in biliopancreatic duct, etc.
Therefore, in our experiment, SAP rats were induced by retrograde minipump injection of 3.5% sodium taurocholate via duodenal papilla using segmental eqidural catheter, which was based on Aho method. The “reflux” of sodium taurocholate due to injection using common injector has been reduced in this experiment where segmental eqidural cathete with diameter consistent with that of biliopancreatic duct was applied. The degree and range of edema, hemorrhage and necrosis due to minipump injection of sodium taurocholate are similar to those of human since this method with even pressure can distribute sodium taurocholate in rat pancreatic tissue evenly. The pancreatic injury due to sodium taurocholate, a cytotoxic substance, is dose dependent. Acute pancreatitis models of various severities can be prepared by controlling the concentration of the drug. 5% concentration of inducing dosage was most frequently used in past reports. After years of study, we found relatively severe pancreatitis models would be induced and many pathological changes of multiple organs were irreversible under this concentration, resulting in short survival time, high mortality and insensitivity to SAP medications or other therapies. Therefore, 3.5% sodium taurocholate (0.1 mL/100 g) was adopted in our study. Certain mortality was observed in model group. The contents of amylase, ascites volume and various inflammatory mediators in plasma were higher in model group than in sham operation group at 3, 6 and 12 h (P < 0.01). Various severities of multiple organ injury were also found in model group. The severity was time dependent. The pathological changes were most significant and severe at 12 h in model group but these reversible changes could be significantly changed after medication. It can be used to simulate the multiple organ injury complicated with SAP patients.
Therefore, this model with pathogenesis, pathological changes, etc similar to clinical SAP is helpful for studies on pathogenesis and therapy of multiple organ injury. This rat SAP inducing method that can induce ideal animal models for empirical studies features small trauma, convenient operation and high achievement ratio. The multiple organ injury occurring at early stage of rat SAP is not severe and reversible. It can be used for evaluation of therapeutic effect. We hope this method could be brought into full play by other researchers.
Severe acute pancreatitis (SAP) is an acute disease, but there is no ideal therapy. It is quite worthwhile to rely on SAP animal model to strength the study on pathogenesis and treatment of SAP.
There are many requirements on an ideal SAP experimental animal model. There should be simple and reliable methods, low cost and high achievement ratio. The pathological changes, progression and response to treatment of the model also should be similar to those of human body.
In this study, the feasibility and significance of the SAP animal model were studied. And the preparation methods for an ideal model of SAP rats with multiple organ injury were established. This model with pathogenesis, pathological changes, etc similar to clinical SAP is helpful for studies on pathogenesis and therapy of multiple organ injury.
This rat SAP inducing method that can induce ideal animal models for empirical studies features small trauma, convenient operation and high achievement ratio. The multiple organ injury occurring at early stage of rat SAP is not severe and reversible. It can be used for evaluation of therapeutic effect. We hope this method could be brought into full play by other researchers.
Severe acute pancreatitis (SAP) is a fatal systemic disease featuring acute onset, serious conditions, high incidence of complications and 20%-30% of mortality.
This is a very interesting paper. Title reflects the major contents of the article. Abstract gives a clear delineation of the research background, objectives, materials and methods, results (including important data) and conclusions. Methods are innovative and systemic. The statistical methods used are appropriate. The results provide sufficient experimental evidences. Discussion is well organized and an overall theoretical analysis is given. The conclusions are scientifically reliable and valuable.
S- Editor Zhu LH L- Editor Alpini GD E- Editor Lu W
| 1. | Aho HJ, Koskensalo SM, Nevalainen TJ. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 258] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Eibl G, Forgacs B, Hotz HG, Buhr HJ, Foitzik T. Endothelin A but not endothelin B receptor blockade reduces capillary permeability in severe experimental pancreatitis. Pancreas. 2002;25:e15-e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Wang X, Jiang W, Zhao G, Du D, Zhou M, Hang Y, Tong C. Mild hypothermia protects against sodium taurocholate (NaTc)-induced acute pancreatitis in rats with adverse effects on serum cytokines. Pancreas. 2005;30:e80-e86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Rakonczay Z, Takács T, Iványi B, Mándi Y, Pápai G, Boros I, Varga IS, Jost K, Lonovics J. The effects of hypo- and hyperthermic pretreatment on sodium taurocholate-induced acute pancreatitis in rats. Pancreas. 2002;24:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Wang D, Jin D, Wu Z, Zou W, Xu D, Zheng Z, Liu X. Therapeutic effects of human interleukin 10 gene transfer on severe acute pancreatitis in rats, an experimental study. Zhonghua YiXue ZaZhi. 2002;82:844-847. [PubMed] |
| 6. | Foitzik T, Hotz HG, Eibl G, Buhr HJ. Experimental models of acute pancreatitis: are they suitable for evaluating therapy? Int J Colorectal Dis. 2000;15:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Cosen-Binker LI, Binker MG, Negri G, Tiscornia O. Experimental model of acute pancreatitis in Wistar rat: glucocorticoid treatment profile. Dig Dis Sci. 2003;48:1453-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Vera-Portocarrero LP, Lu Y, Westlund KN. Nociception in persistent pancreatitis in rats: effects of morphine and neuropeptide alterations. Anesthesiology. 2003;98:474-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Uruñuela A, Manso MA, de la Mano AM, Sevillano S, Orfao A, de Dios I. Asynchronous impairment of calcium homoeostasis in different acinar cells after pancreatic duct obstruction in rat. Clin Sci (Lond). 2002;102:615-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Jin C, Li JC. Create the mouse model of severe acute pancreatitis induced by caerulein plus lipopolysaccharide and study on its pathogenesis. ShiYan ShengWu XueBao. 2003;36:91-98. [PubMed] |
| 11. | Yang R, Uchiyama T, Alber SM, Han X, Watkins SK, Delude RL, Fink MP. Ethyl pyruvate ameliorates distant organ injury in a murine model of acute necrotizing pancreatitis. Crit Care Med. 2004;32:1453-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Tomita Y, Kuwabara K, Furue S, Tanaka K, Yamada K, Ueno M, Ono T, Maruyama T, Ajiki T, Onoyama H. Effect of a selective inhibitor of secretory phospholipase A2, S-5920/LY315920Na, on experimental acute pancreatitis in rats. J Pharmacol Sci. 2004;96:144-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Gülçubuk A, Sönmez K, Gürel A, Altunatmaz K, Gürler N, Aydin S, Oksüz L, Uzun H, Güzel O. Pathologic alterations detected in acute pancreatitis induced by sodium taurocholate in rats and therapeutic effects of curcumin, ciprofloxacin and metronidazole combination. Pancreatology. 2005;5:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Mozo G, del Olmo ML, Caro-Patón A, Reyes E, Manzano L, Belmonte A, Alvarez-Mon M. Lung changes and cytokine levels in a model of experimental acute pancreatitis. Rev Esp Enferm Dig. 2002;94:53-66. [PubMed] |
| 15. | Balachandra S, Genovese T, Mazzon E, Di Paola R, Thiemerman C, Siriwardena AK, Cuzzocrea S. Inhibition of tyrosine-kinase-mediated cellular signaling by tyrphostins AG 126 and AG556 modulates murine experimental acute pancreatitis. Surgery. 2005;138:913-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Hegyi P, Rakonczay Z, Sári R, Góg C, Lonovics J, Takács T, Czakó L. L-arginine-induced experimental pancreatitis. World J Gastroenterol. 2004;10:2003-2009. [PubMed] |
| 17. | Strate T, Mann O, Kleinhans H, Schneider C, Knoefel WT, Yekebas E, Standl T, Bloechle C, Izbicki JR. Systemic intravenous infusion of bovine hemoglobin significantly reduces microcirculatory dysfunction in experimentally induced pancreatitis in the rat. Ann Surg. 2003;238:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Yasuda T, Takeyama Y, Ueda T, Takase K, Nishikawa J, Kuroda Y. Splenic atrophy in experimental severe acute pancreatitis. Pancreas. 2002;24:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Paszkowski AS, Rau B, Mayer JM, Möller P, Beger HG. Therapeutic application of caspase 1/interleukin-1beta-converting enzyme inhibitor decreases the death rate in severe acute experimental pancreatitis. Ann Surg. 2002;235:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Wang X, Wang B, Wu J, Wang G. Beneficial effects of growth hormone on bacterial translocation during the course of acute necrotizing pancreatitis in rats. Pancreas. 2001;23:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Kinnala PJ, Kuttila KT, Grönroos JM, Havia TV, Nevalainen TJ, Niinikoski JH. Splanchnic and pancreatic tissue perfusion in experimental acute pancreatitis. Scand J Gastroenterol. 2002;37:845-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |