Published online Sep 14, 2007. doi: 10.3748/wjg.v13.i34.4554
Revised: June 2, 2007
Accepted: June 9, 2007
Published online: September 14, 2007
AIM: To investigate the expressions of PTEN, PPM1A and P-Smad2 in hepatocellular carcinoma (HCC) and their significance.
METHODS: The expressions of PTEN, PPM1A and P-Smad2 in 31 HCC tissues, 25 adjacent liver tissues and 13 non-tumor liver tissues were detected by using Envision immunohistochemical technique.
RESULTS: The positive expression (64.52%) and staining intensity (4.19 ± 3.31) of PTEN in the cytoplasm of HCC were significantly lower and weaker than those in the adjacent or non-tumor liver tissues (97.37%, 7.88 ± 0.93; 100%, 7.77 ± 0.93, respectively) (P < 0.05), and its staining intensity in the cytoplasm of HCC, which belongs to Edmondson pathologic grades II-III and above, was also lower than that of gradeIandI-II. Furthermore, its location in the nucleus or cytoplasm of liver cells was negatively correlated with the progression of liver disease (r = -0.339, P = 0.002); most of PPM1A might be only expressed in the nucleus of adjacent liver tissues, non-HCC tissues or Edmondson gradeIandI-II HCC, but it was mainly expressed in the cytoplasm of HCC with Edmondson grade ≥ II, weakly or negatively expressed in the nucleus (P < 0.05), and its location was negatively correlated with the progression of liver disease (r = -0.45, P = 0.0000). P-Smad2, which was mostly located in the nucleus and cytoplasm of gradeIandI-II HCC, surrounding or non-tumor liver tissues, was only in the nucleus of HCC with Edmondson grade II and above (P < 0.001), and its location was positively correlated with the disease progression (r = 0.224, P = 0.016). Spearman correlation analysis revealed that P-Smad2 was significantly negatively correlated with PTEN and PPM1A (r = -0.748, P = 0.000; r = -0.366, P = 0.001, respectively); and PTEN and PPM1A were positively correlated with HCC carcinogenesis (r = 0.428, P = 0.000).
CONCLUSION: The aberrant location of expression and staining intensity of PTEN, PPM1A and P-Smad2 in HCC and their relationship might have an impact on the pathogenesis of HCC.
- Citation: Wu SK, Wang BJ, Yang Y, Feng XH, Zhao XP, Yang DL. Expression of PTEN, PPM1A and P-Smad2 in hepatocellular carcinomas and adjacent liver tissues. World J Gastroenterol 2007; 13(34): 4554-4559
- URL: https://www.wjgnet.com/1007-9327/full/v13/i34/4554.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i34.4554
Hepatocellular carcinoma (HCC) is the 5th most common solid tumor worldwide and accounts for about 110 000 deaths each year in China, with the 2nd most common cause of mortality among all malignant tumors[1]. The pathogenesis of HCC is not clearly elucidated so far; hence, the exploration of the key genes and pathways involved in hepatocarcinogenesis and novel therapeutics strategies is very crucial. Many data indicated that TGF-β/Smads pathway significantly acted on the pathogenesis of HCC[2,3]. Recent studies showed persistent accumulation of phosphorylated Smads (P-Smads) in the nucleus of HCC may be associated with the development of HCC. But its dephosphorylation regulation remains unknown[4]. Lin et al[5] identified PPM1A as a protein serine/threonine phosphatase which can dephosphorylate and promote nuclear export of TGF-β-activated Smad2/3, and implied that dual specific phosphatases (DUSPs) also catalyze dephosphorylation of pS/Ts. Phosphatase and tensin homolog deleted on chromosome ten (PTEN) is the first tumor suppressor gene which can encode a dual specificity phosphatase (protein phosphatase and lipid phosphatase), its deletion or mutation may impact on the development and prognosis of HCC and other tumors[6-8]. In the present study, we investigated the expressions of PTEN, PPM1A and P-Smad2 in HCC with different Edmondson pathological grades by using immunohistochemical technique and analyzed their possible relationship associated with the pathogenesis of HCC.
This study included 31 HCC and 25 corresponding paracancerous tissues and 13 non-tumor liver tissues (5 liver cirrhosis, 3 hepatitis and 5 normal liver tissues) obtained from Tongji Hospital from 1997 to 1998 and 2003 to 2004. Among 31 HCC cases, 27 were males and 4 were females, with age ranged from 24-78 (mean, 43.45 ± 10.77) years. None of the cases had distant metastases and received any chemotherapy and radiotherapy. According to Edmondson Grading System, 10 cases belonged to gradeIandI-II, 7 cases grade II and 14 cases > grade II. In addition, among 25 cases of paracancerous liver tissues, 11 cases had intrahepatic vascular embolism and 14 cases had not. All tissue samples were fixed in formalin and imbedded in paraffin, and cut into 4-μm thick sections.
Rabbit anti-phosphorylated Smad2 (P-Smad2) monoclonal antibody (1:100 dilution), mouse anti-PPM1A monoclonal antibody (1:25 dilution) and rabbit anti-human PTEN polyclonal antibody (1:400 dilution) were purchased from CellSignaling Technology Company, Abcam and Zhongshan Biotechnology Company, Beijing. DAKO Envision kit was purchased from DAKO Company.
Immunohistochemical staining was performed by using DAKO Envision (DAKO, Carpinteria, CA) kit according to the manufacturer’s instructions. Briefly, each section was deparaffinized, rehydrated and incubated with fresh 3 mL/L hydrogen peroxide in methanol for 30 min at room temperature and then washed in phosphate-buffered saline (PBS). Sections were incubated with normal goat serum for 30 min to block the nonspecific antibody binding. Sections were then incubated with primary antibody against P-Smad2 or PTEN or PPM1A overnight at 4°C, washed three times in PBS, followed by incubation with respective secondary antibody for 30 min at room temperature. Then 3, 3’-diaminobenzidine tetrachloride (DAB) was used for color development and the sections were counterstained with hematoxylin. Primary antibody was substituted by PBS for negative control.
The degree of staining was categorized by the extent and intensity of the straining; the staining results were assessed according to Detre et al[9]. Briefly, the cells staining positively throughout the section were assigned scores from 1 to 6 as follows: 1 = 0%-4%; 2 = 5%-19%; 3 = 20%-39%; 4 = 40%-59%; 5 = 60%-79%; and 6 = 80%-100%. The average intensity, corresponding to the presence of negative, weak, intermediate, and strong staining, was given a score from 0 to 3, respectively. The positive cell score was added to the average intensity to form an additive quickscore. A positive cut-off quick score was ≥ 3.
Data analyses were performed with the SPSS (version 12.0) statistical package on a personal computer. Significant differences between variables were assessed by Student’s t test, Chi-square test or Fisher’s exact probability test, when appropriate. The correlation between factors was evaluated by using Spearman rank correlation coefficient test. A P value less than 0.05 was considered statistically significant.
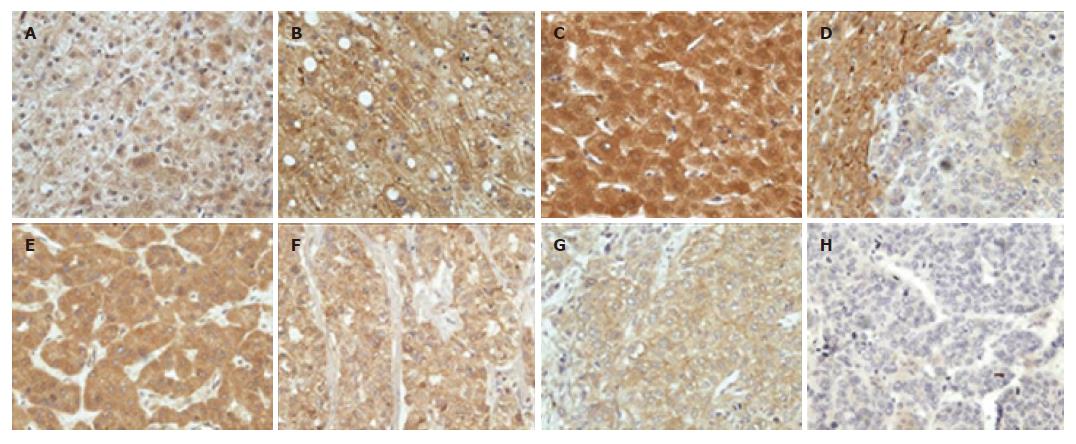
The expression of PTEN was localized generally in the nucleus, cytoplasm and cell membrane of liver cells (Figure 1), but its distribution and staining intensity in HCC, paracancerous or non-tumor liver tissues (including normal liver tissues, hepatitis tissue and cirrhotic liver tissues) were different. As shown in Table 1, 20 of 31 (64.52%) HCCs tissues were positive for PTEN, mainly expressed in the cytoplasm and cell membrane. PTEN expression was strongly positive in 13 of 17 HCCs with Edmondson gradeIandI-II, but weakly positive in 8 of 14 HCC with grade II-III and above. Furthermore, PTEN was stained strongly in the cytoplasm, nucleus and/or cell membrane of 24 of 25 paracancerous and 13 non-tumor liver tissues compared with HCC tissues (P < 0.05).
| Parameters | Cases (n) | Positive cases | Mean score of PTEN staining (mean ± SD) | ||
| Nucleus | Cytoplasm | Cell membrane | |||
| HCC (Edmondson grading system) | 31 | 20ac | 0abc | 4.19 ± 3.31abc | 4.77 ± 3.92 |
| GradeIandI-II | 10 | 8 | 0 | 5.80 ± 3.12e | 5.30 ± 3.68 |
| Grade II | 7 | 4 | 0 | 4.14 ± 3.89 | 4.86 ± 4.56 |
| Grade II-III and above | 14 | 8 | 0 | 3.07 ± 2.87e | 4.36 ± 4.01 |
| Paracancerous tissue | 25 | 24 | 3.88 ± 3.14ab | 7.88 ± 0.93abd | 5.16 ± 3.69d |
| Non-tumor liver tissue | 13 | 13 | 5.79 ± 2.31bc | 7.77 ± 0.93bcd | 3.92 ± 3.35d |
| Cirrhotic liver tissue | 5 | 5 | 7.40 ± 1.14 | 8.40 ± 0.55 | 4.40 ± 4.10 |
| Hepatitis tissue | 3 | 3 | 4.33 ± 3.79 | 8.00 ± 1.00 | 4.33 ± 3.79 |
| Normal liver tissue | 5 | 5 | 5.0 ± 1.41 | 7.00 ± 0.71 | 3.20 ± 2.95 |
| Intrahepatic vascular embolism | |||||
| Present | 11 | 5 | 0b | 3.00 ± 3.46be | 3.27 ± 3.79 |
| Absent | 14 | 12 | 0b | 6.28 ± 2.37be | 6.71 ± 2.89 |
In addition, compared with the group without intrahepatic vascular embolism (85.71%, 12/14), PTEN was weakly positive in HCC with intrahepatic vascular embolism (45.45%, 5/11). Spearman rank correlation analysis showed that PTEN expression was negatively correlated with intrahepatic vascular embolism (r = -0.428, P = 0.033).
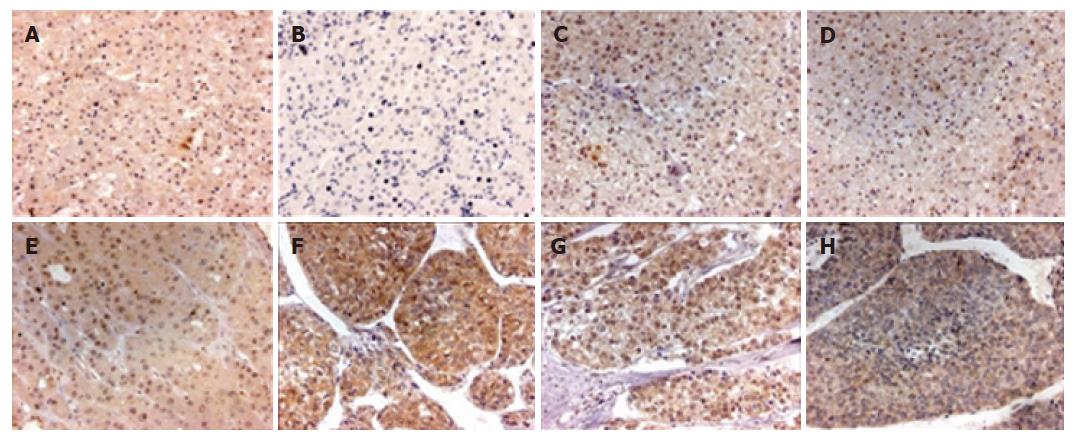
We found that PPM1A staining was mainly localized in the nucleus and cytoplasm of different type liver tissues (Figure 2), but its distribution and staining intensity were different. PPM1A was strongly expressed in the nucleus of non-tumor liver tissues, paracancerous liver tissues and gradeIandI-II HCC, with weak or negative expression in the cytoplasm; whereas strongly expressed in the cytoplasm of HCC with grades II-III and above, with weak expression in the nucleus, thereby showing a significant difference in distribution and staining intensity (P < 0.001). However, Spearman rank correlation analysis showed that the location and staining intensity of PPM1A in HCC were not correlated with intrahepatic vascular embolism (r = -0.038, P = 0.821) (Table 2).
| Parameters | Cases (n) | Mean score of PPM1A staining (mean ± SD) | |
| Nucleus | Cytoplasm | ||
| HCC (Edmondson grades) | 31 | 3.45 ± 3.00abc | 6.32 ± 3.00abc |
| GradeIandI-II | 10 | 5.60 ± 2.54e | 3.90 ± 4.14e |
| Grade II | 7 | 3.57 ± 2.15b | 7.86 ± 0.69 |
| Grade II-III and above | 14 | 1.86 ± 2.79d,b | 7.28 ± 1.38be |
| Paracancerous tissue | 25 | 6.28 ± 1.95ab | 2.08 ± 3.45ab |
| Non-tumor liver tissue | 13 | 5.38 ± 1.45be | 0cb |
| Liver cirrhosis | 5 | 6.40 ± 0.55 | 0 |
| Hepatitis | 3 | 3.33 ± 0.58 | 0 |
| Normal liver tissue | 5 | 5.60 ± 1.14 | 0 |
| Intrahepatic vascular embolism | |||
| Present | 11 | 3.82 ± 3.22 | 6.09 ± 3.11 |
| Absent | 14 | 3.71 ± 3.05 | 6.14 ± 3.44 |
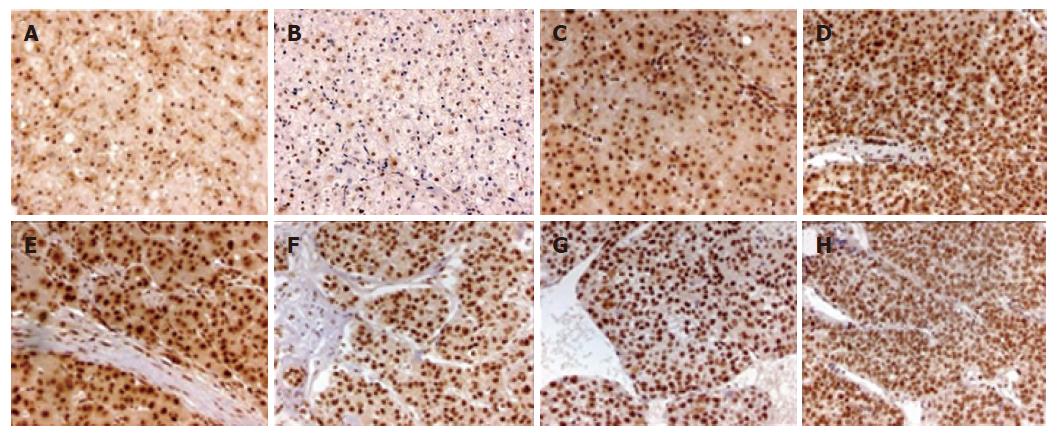
P-Smad2 staining was mostly localized in the nucleus and its surrounding cytoplasm (Figure 3), with different pattern of its distribution in different types of liver tissues (Table 3). P-Smad2 was expressed in the nucleus and the surrounding cytoplasm of non-tumor liver tissues, paracancerous liver tissues and HCC < grade II, and only in the nucleus of ≥ grade II HCC. Although its staining intensity in the nucleus was not significantly different between HCC and paracancerous tissues, P-Smad2 expression was stronger in the nucleus and weaker in the cytoplasm of HCC than those of non-tumor liver tissues (P < 0.05). In addition, the expression of P-Smad2 in HCC was not associated with intrahepatic vascular embolism (r = 0.052, P = 0.775).
| Parameters | Cases (n) | Mean score of P-Smad2 staining (mean ± SD) | |
| Nucleus | Cytoplasm | ||
| HCC (Edmondson grades) | 31 | 8.61 ± 0.68bd | 1.74 ± 3.08abd |
| GradeIandI-II | 10 | 8.70 ± 0.48 | 4.90 ± 3.54c |
| Grade II | 7 | 7.85 ± 1.07 | 0.71 ± 1.89c |
| Grade II-III and above | 14 | 8.79 ± 0.58 | 0d |
| Paracancerous tissue | 25 | 8.28 ± 0.74b | 7.28 ± 1.21ab |
| Non-tumor liver tissue | 13 | 7.08 ± 1.61bd | 5.31 ± 2.84bd |
| Cirrhosis tissue | 5 | 8.40 ± 0.55c | 7.40 ± 1.81 |
| Hepatitis tissue | 3 | 5.33 ± 0.58c | 1.33 ± 2.31 |
| Normal liver tissue | 5 | 6.80 ± 1.64 | 5.60 ± 0.89 |
| Intrahepatic vascular embolism | |||
| Present | 11 | 8.82 ± 0.40 | 2.36 ± 3.35 |
| Absent | 14 | 8.43 ± 0.76 | 2.00 ± 3.37 |
The expressions of PTEN, PPM1A and P-Smad2 in HCC were not related with age and gender, but might be associated with progression of liver disease. With liver pathology developing from non-tumor liver tissues, adjacent cancerous tissues to HCC, the location of PTEN shifted from the nucleus to the cytoplasm or cell membrane, even some negative staining. Most of PPM1A also shifted from the nucleus to the cytoplasm. But P-Smad2 was translocated from the nucleus and cytoplasm to the nucleus accumulation. Spearman rank correlation analysis suggested that the staining location of PTEN and PPM1A in liver cells was negatively correlated with progression of liver disease (r = -0.339, P = 0.002; r = -0.45, P = 0.0000, respectively), but P-Smad2 was positively correlated (r = 0.228, P = 0.015) (Table 4). As shown in Table 5, with progression of tumor Edmondson grades, the expression of PPM1A in the nucleus was decreased (r = -0.322, P = 0.029), but P-Smad2, in more cases, was accumulated in the nucleus (r = 0.459, P = 0.003), implying that although the location of PTEN was positively correlated with that of PPM1A in HCC (r = 0.428, P = 0.000), the expression and location of P-Smad2 were significantly negatively correlated with those of PTEN and PPM1A (r = -0.748, P = 0.000; r = -0.366, P = 0.001, respectively).
| Types | Cases (n) | PTEN | PPM1A | P-Smad2 | |||
| Cytoplasm | Nucleus | Cytoplasm | Nucleus | Cytoplasm | Nucleus | ||
| HCC | 31 | 20 | 0 | 26 | 20 | 8 | 31 |
| Paracancerous tissue | 25 | 24 | 17 | 7 | 25 | 25 | 25 |
| Non-tumor liver tissue | 13 | 13 | 12 | 0 | 13 | 11 | 13 |
| Pathological type | Cases (n) | PPM1A | P-Smad2 | ||
| Cytoplasm | Nucleus | Cytoplasm | Nucleus | ||
| HCC (Edmondson grade) | 31 | 26 | 20 | 8 | 31 |
| GradeIandI-II | 10 | 5 | 9 | 7 | 10 |
| Grade II | 7 | 7 | 6 | 1 | 7 |
| Grade II-III and above | 14 | 14 | 5 | 0 | 14 |
Phosphatase and tensin homolog deleted on chromosome ten (PTEN) was discovered as a tumor suppressor gene in 1997. It could regulate normal cell growth by negatively regulating the phosphatidylinositol 3-kinase (PI3K) signaling pathway which is an important driver of cell proliferation and cell survival, and its deletion, mutation or otherwise inactivation was frequently correlated with the development and progression of many human tumors[6,7,10]. In this study, we detected the expression of PTEN in 31 HCCs, 25 paracancerous tissues and 13 non-tumor liver tissues by using immunohistochemical technique. Our results showed that the positive rates of PTEN in HCC, paracancerous and non-tumor liver tissues were 61.29%, 97.37% and 100%, respectively. Furthermore, the staining intensity of PTEN in the cytoplasm of HCC was significantly weaker than that of paracancerous and non-tumor liver tissues. In addition, the staining intensity of PTEN in the cytoplasm of HCC with grade ≥ II-III was weaker compared to HCC with gradeIandI-II. Also, PTEN expression in HCC was correlated with intrahepatic vascular embolism. These results are in agreement with previous studies[8,11] and imply that the expression and intensity of PTEN maybe related to the malignant and invasive potential of HCC, and its deletion and weak expression would result in tumorigenesis and development of HCC.
In this study, we also observed that PTEN was localized in the nucleus, cytoplasm or cell membrane of non-tumor or paracancerous liver tissues, but mainly localized in the cytoplasm or cell membrane of HCC, and the nuclei were almost negative, suggesting that its location was closely correlated with the progression of liver disease. However, whether this phenomenon is connected with the role of PTEN in tumor suppressionneeds to be further studied.
PPM1A is a member of the PP2C family of Ser/Thr protein phosphatases which are known to be negative regulators of cell stress response pathways, such as p38 and JNK kinase cascades. This phosphatase can also dephosphorylate cyclin-dependent kinases, thus may be involved in cell cycle control. Over-expression of the phosphatase is reported to activate the expression of the tumor suppressor gene TP53/p53, which leads to G2/M cell cycle arrest and apoptosis[12]. In this experiment, we examined the expression of PPM1A in HCC, paracancerous liver tissues and non-tumor liver tissues. The results showed that PPM1A was located in the nucleus and cytoplasm of liver cells. With the progression of liver disease, most of PPM1A shifted from the nucleus to the cytoplasm and the staining intensity was obviously different, suggesting that its expression location was negatively correlated with liver pathological type (r = -0.45, P = 0.0000) and implying that the location shift of PPM1A may have impact on the carcinogenesis and progression of HCC.
TGF-β/Smads signaling is an important regulator of cell growth pathway, which modulates diverse cellular processes including cell proliferation, differentiation, adhesion, extracellular matrix remodeling, apoptosis and immunomodulation. Smad2 is an essential intracellular transducer for TGF-β signals. In response to TGF-β stimulation, it is phosphorylated by TGF-βIreceptor and forms a complex with Smad3, Smad4 and transported into the nucleus, where Smads cooperates with specific DNA-binding transcription factor CRB/P300 and activates TGF-β signals to regulate gene transcription, inhibit cell growth and promote apoptosis. Xu et al[13] reported that Smad2 phosphorylation or dephosphorylation would play a key role in regulation TGF-β signaling[13]. A recent study showed that phosphorylated R-Smad2 was accumulated in the nucleus of hepatoma cell which might be correlated with hepatocellular tumorigenesis and development[4]. In this study, we found phosphorylated Smad2 was strongly expressed in the nucleus and cytoplasm of non-tumor liver tissues, paracancerous liver tissues and HCC with Edmondson gradeIandI-II. However, P-Smad2 was only located in the nucleus of HCC ≥ grade II, implying that accumulation of P-Smad2 in the nucleus was closely correlated with liver pathological type and progression of hepatocarcinogenesis and may play an important role in HCC development.
Spearman rank correlation analysis results suggested that the expression location of PTEN and PPM1A in the nucleus or cytoplasm of HCC was negatively correlated with P-Smad2. Lin et al[5] demonstrated that PPM1A/PP2C can dephosphorylate the SXS motif of Smads, and nucleus-to-cytoplasm shift of PPM1A would result in P-Smad2 accumulation in the nucleus and TGF-β signaling termination[5]. Hence, we presumed that PTEN, which is dual specific phosphatases (DUSPs), may also catalyze dephosphorylation of pS/Ts of Smad2 in the nucleus or cooperate with PPM1A, and its nucleus-to-cytoplasm shift, or weak or negative expression would block TGF-β signaling, deregulate hepatocellular growth and promote tumorigenesis and development of HCC. However, further studies are needed to clarify it.
In conclusion, deletion or weak expression or nucleocytoplasmic shift of PTEN and PPM1A and accumulation of P-Smad2 in the nucleus of hepatoma cells might be associated with the tumorigenesis and progression of primary hepatocellular carcinoma.
Co-correspondence: Dr. Xi-Ping Zhao
S- Editor Liu Y L- Editor Kumar M E- Editor Wang HF
| 1. | Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis. 2005;25:143-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 311] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 2. | Saffroy R, Pham P, Lemoine A, Debuire B. Molecular biology and hepatocellular carcinoma: current status and future prospects. Ann Biol Clin (Paris). 2004;62:649-656. [PubMed] |
| 3. | Bissell DM. Chronic liver injury, TGF-beta, and cancer. Exp Mol Med. 2001;33:179-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Hua YP, Huang JF, Liang LJ, Li SQ, Lai JM, Liang HZ. The study of inhibition effect of octreotide on the growth of hepatocellular carcinoma xenografts in situ in nude mice. Zhonghua WaiKe ZaZhi. 2005;43:721-725. [PubMed] |
| 5. | Lin X, Duan X, Liang YY, Su Y, Wrighton KH, Long J, Hu M, Davis CM, Wang J, Brunicardi FC. PPM1A functions as a Smad phosphatase to terminate TGFbeta signaling. Cell. 2006;125:915-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 397] [Cited by in RCA: 394] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 6. | Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3525] [Cited by in RCA: 3582] [Article Influence: 127.9] [Reference Citation Analysis (0)] |
| 7. | Myers MP, Stolarov JP, Eng C, Li J, Wang SI, Wigler MH, Parsons R, Tonks NK. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci USA. 1997;94:9052-9057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 620] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 8. | Fujiwara Y, Hoon DS, Yamada T, Umeshita K, Gotoh M, Sakon M, Nishisho I, Monden M. PTEN / MMAC1 mutation and frequent loss of heterozygosity identified in chromosome 10q in a subset of hepatocellular carcinomas. Jpn J Cancer Res. 2000;91:287-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Detre S, Saclani Jotti G, Dowsett M. A "quickscore" method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48:876-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 680] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 10. | Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2004] [Cited by in RCA: 2036] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 11. | Yang Z, Yi J, Li X, Long W. Correlation between loss of PTEN expression and PKB/AKT phosphorylation in hepatocellular carcinoma. J Huazhong Univ Sci Technolog Med Sci. 2005;25:45-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Saadat M, Kikuchi K. Assignment of the gene encoding magnesium-dependent protein phosphatase 1alpha (PPM1A) to human chromosome 14q22--& gt; q23 and rat chromosome 6q24 by fluorescence in situ hybridization. Cytogenet Genome Res. 2005;108:363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Xu L, Kang Y, Cöl S, Massagué J. Smad2 nucleocytoplasmic shuttling by nucleoporins CAN/Nup214 and Nup153 feeds TGFbeta signaling complexes in the cytoplasm and nucleus. Mol Cell. 2002;10:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 192] [Article Influence: 8.3] [Reference Citation Analysis (0)] |