Published online Sep 7, 2007. doi: 10.3748/wjg.v13.i33.4526
Revised: May 12, 2007
Accepted: May 21, 2007
Published online: September 7, 2007
Hepatocellular carcinoma (HCC) metastasizes to the mandible is infrequently seen. Solitary bony metastasis to the mandible is rarer. The intractable bleeding caused by rupture of the metastatic HCC is challenging to clinicians. We present a case of a 74-year-old woman with HCC under control without progression for 3 years. Left facial swelling and episodes of bleeding developed recently and biopsy revealed a metastatic HCC. Computer tomography showed a large tumor in parapharyngeal space with evident mandibular ramus destruction. Bleeding occurred from the metastatic tumor but could not be controlled by electrocauterization, Surgicel™, tissue glue, and bone wax and angiographic embolization. Palliative radiotherapy (2400 cGy in 6 fractions) was tried and the intractable bleeding was successfully stopped after the radiotherapy. Because of the hypervascular and osteolytic nature of the solitary mandibular metastatic lesion, the bleeding was troublesome. Radiotherapy provided successful control of intractable bleeding from the metastatic tumor.
- Citation: Huang SF, Wu RC, Chang JTC, Chan SC, Liao CT, Chen IH, Yeh CN. Intractable bleeding from solitary mandibular metastasis of hepatocellular carcinoma. World J Gastroenterol 2007; 13(33): 4526-4528
- URL: https://www.wjgnet.com/1007-9327/full/v13/i33/4526.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i33.4526
Hepatocellular carcinoma (HCC), with an estimated 1 million cases per year, is the 5th most common cancer in the world, particularly in the Southeast Asia[1]. HCC with extrahepatic metastasis has been reported in approximately 50% of cases; lungs, abdominal lymphatics, adrenal glands, great veins adjacent to the liver, the diaphragm, or the skeleton could be involved. Bone metastasis in HCC has been reported in 10.1% of patients, with the vertebrae being the most frequently affected, followed by (in decreasing order) ribs, sternum, and pelvis[2]. The mandible is a rare site of HCC metastasis. Here we report a rare case of mandibular metastasis of HCC with intractable bleeding and the bleeding was controlled successfully by radiotherapy.
A 74-year-old woman was suffering from chronic hepatitis C-related HCC for 6 years. Segmental hepatectomy was done initially. She had undergone transcatheter arterial chemoembolization (TACE) 9 times, and percutaneous ethanol injection (PEI) 3 times for tumor recurrence. She had 3 years of progression-free survival.
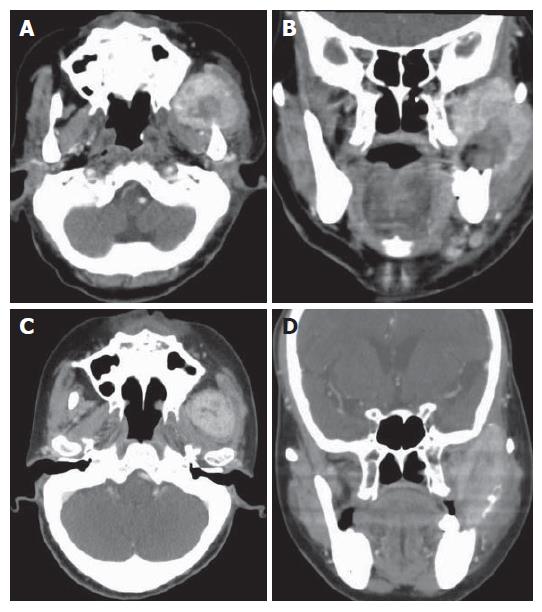
She was referred to our clinic due to a 1 cm × 1 cm ulcerative mass in the left buccal region, with preauricular swelling for 2 wk. Biopsy was done over the buccal mass. Two days later, she presented in the emergency room with the chief complaint of progressive facial swelling and persistently blood oozing from the left buccal tumor. The laboratory tests were AST (GOT) 58 U/L, ALT (GPT) 49 U/L and platelet count 179 × 103/μL. Her liver disease was in the status of Child-Pugh class A. CT scan demonstrated a 6.2 cm × 5.0 cm osteolytic lesion in the left parapharyngeal space with destruction of the left mandible (Figure 1A). The lesion extended from the mandibular ramus to temporal muscle space (Figure 1B). Diffuse and massive bleeding occurred from the ulcerative mass the next morning after admission. Hemorrhage could not be stopped by electrocauterization or suture ligation alone, but it was stopped temporarily by the use of electrocauterization, Surgicel™, tissue glue, and bone wax concomitantly after 700 mL blood loss. The mandibular lesion was proved pathologically to be a metastatic HCC. Bone scan showed the mandibular lesion was a solitary metastasis of HCC. CT scan of the brain, chest, abdomen and pelvis failed to reveal any evidence of metastatic disease.
Unfortunately, a second episode of hemorrhage occurred 5 d later. Due to antecedent experience of difficult hemostasis, we tried vascular embolization. Under angiography, the injection of contrast medium through the left common carotid artery and external carotid artery showed a hypervascular stain about 3.8 cm in the left masticator space. The left maxillary artery and the main feeding artery were occluded by the injection of permanent embolizer (500 to 700 U of polyvinyl alcohol particles, PVA). Over 90% of tumor stain was obliterated after embolization and the main feeding artery was occluded. However, slow but profuse oozing from the left buccal wound persisted. After discussion with radiation oncologist, palliative radiotherapy was suggested in the handful treatment options. We arranged radiotherapy for the left mandibular metastatic lesion (2400 cGy in 6 fractions). Oozing decreased about 3 d after the start of radiotherapy and stopped 5 d after the completion of radiotherapy and the tumor shrunk after radiotherapy (Figure 1C and D). Now the woman was stable with disease at a follow-up period of 5 mo.
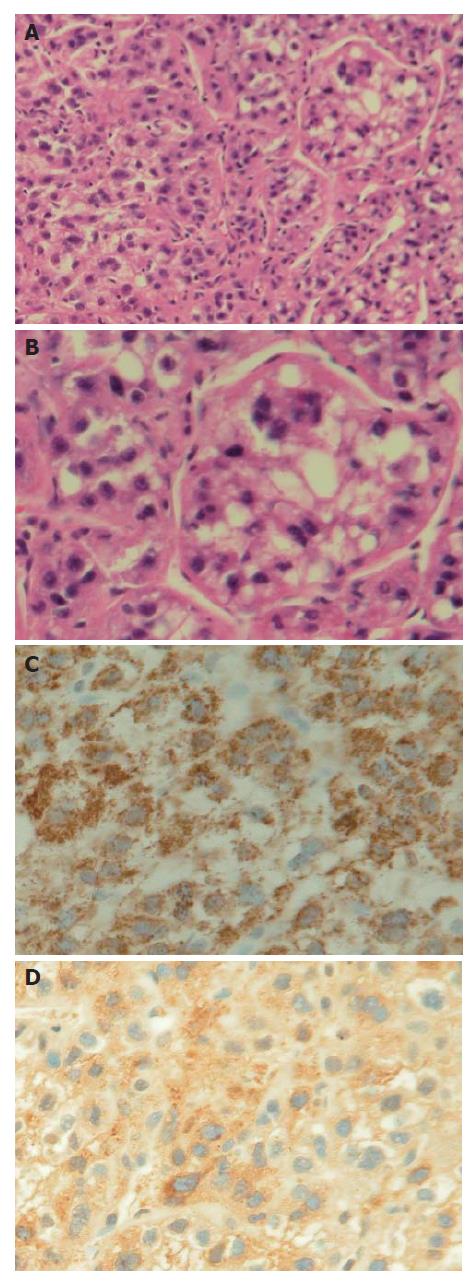
Histopathological examination of the ulcerative mass at the mandible revealed the metastasis was from well-differentiated HCC (Figure 2A and B). Neoplastic cells were diffusely stained by hepatocyte-paraffin-1 (Hep Par-1) (Figure 2C) and focally positive for α-fetoprotein (AFP) (Figure 2D).
With advances in surgical techniques and an improvement in perioperative care, surgical mortality rates in HCC patients receiving hepatectomy have reduced significantly. Postoperative recurrence is universally high and remains the main cause of late deaths. The development of PEI and TACE contributes to the better local control of HCC. Nevertheless, distant metastasis of HCC is more and more frequently seen after the better control of primary disease. The most common sites of HCC metastasis are the lungs, bones, brain, and skin[3]. The mode of extrahepatic spread from HCC is usually hematogenous metastasis. The incidence of bone metastasis from HCC accounts for approximately 1.6%-16% and the most common sites are the vertebrae, followed by pelvis and ribs[4]. Mandible is rarely metastasized by HCC. In addition, bone metastasis usually manifests as multiple lesions. Solitary bony metastasis in HCC is infrequently met, as in our patient.
Two modes of spread were ever proposed for the tumor spreading from the liver to the maxillofacial territory. The metastatic dissemination reaches the lungs first, and possibly the maxillofacial area later through the communication between the hepatic artery and portal vein. It has been postulated that there is a connection between the azygos and hemiazygos veins and the vertebral venous plexus (Batson’s plexus)[2,5]. There is the existence of free communication between the neck, thorax, abdomen and pelvis venous systems with the non-valve vertebral venous plexus that extends from cranial base to coccyx. Any pressure increment inside the abdomen can create an ascendant flow through the vertebral venous plexus. The HCC cells could reach maxillofacial territory through these two hematogenous connections and grow into a metastatic lesion in the mandibular region.
Any malignancy in head and neck, either primary or metastatic lesions, can manifest as bleeding. However, HCC metastatic lesions, as seen in our case, are hypervascular and osteolytic in nature. It may rupture spontaneously to cause hemorrhage and could be devastating. Chen et al[6] reported a case of life-threatening hemorrhage from a sternal metastasis of HCC. The acute hemorrhage in mandibular metastasis of HCC was ever reported to be managed by Surgicel™ (Johnson & Johnson, New Brunswick, NJ) and bone wax packing[7]. In our patient, three methods were utilized to stop bleeding: electrocauterization, Surgicel™, tissue glue and bone wax in the 1st episode, but failed. Embolization was used in the 2nd hemorrhagic episode and failed again. Hypervascularity of the tumor and no single identifiable bleeder partly explained the reason of failure in two earlier methods. Palliative radiotherapy (2400 cGy) successfully stopped the bleeding and shrunk the tumor. The mechanisms behind hemostasis after radiation therapy were decrease of the tumor size, the tumoral vascularity, and induced fibrosis in the peripheral osseous structure. Surgery for the solitary mandibular metastasis of HCC was not considered in this patient. The metastatic lesion in our patient was osteolytic and invaded into the temporal muscle space. The lesion located in proximity of the skull base and was considered inoperable.
In conclusion, we reported a rare case of solitary mandibular metastasis of HCC and the intractable bleeding from the metastatic lesion was controlled successfully by palliative radiation therapy. Palliative therapy could be an option when coping with refractory bleeding in head and neck malignancies.
S- Editor Zhu LH L- Editor Kumar M E- Editor Lu W
| 1. | Kao JH, Chen DS. Changing disease burden of hepatocellular carcinoma in the Far East and Southeast Asia. Liver Int. 2005;25:696-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Yoshimura Y, Matsuda S, Naitoh S. Hepatocellular carcinoma metastatic to the mandibular ramus and condyle: report of a case and review of the literature. J Oral Maxillofac Surg. 1997;55:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Lee YT, Geer DA. Primary liver cancer: pattern of metastasis. J Surg Oncol. 1987;36:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Fukutomi M, Yokota M, Chuman H, Harada H, Zaitsu Y, Funakoshi A, Wakasugi H, Iguchi H. Increased incidence of bone metastases in hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2001;13:1083-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 146] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Batson OV. The function of the vertebral veins and their role in the spread of metastases. 1940. Clin Orthop Relat Res. 1995;4-9. [PubMed] |
| 6. | Chen CY, Chau GY, Yen SH, Hsieh YH, Chao Y, Chi KH, Li CP, Chang FY, Lee SD. Life-threatening haemorrhage from a sternal metastatic hepatocellular carcinoma. J Gastroenterol Hepatol. 2000;15:684-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |