Published online Sep 7, 2007. doi: 10.3748/wjg.v13.i33.4509
Revised: April 23, 2007
Accepted: April 26, 2007
Published online: September 7, 2007
AIM: To evaluate the effect of preoperative transcatheter arterial chemoembolization (TACE) on proliferation of hepatocellular carcinoma (HCC) cells.
METHODS: A total of 136 patients with HCC underwent liver resection. Of 136 patients, 79 patients received 1 to 5 courses of TACE prior to liver resection (TACE group), who were further subdivided into four groups: Group A (n = 11) who received 1 to 4 courses of chemotherapy alone; Group B (n = 33) who received 1 to 5 courses of chemotherapy combined with iodized oil; Group C (n = 23) who received 1 to 3 courses of chemotherapy combined with iodized oil and gelatin sponge; and Group D (n = 12) who received 1 to 3 courses of chemotherapy combined with iodized oil, ethanol and gelatin sponge. The other 57 patients only received liver resection (non-TACE group). The expressions of Ki-67 and proliferating cell nuclear antigen (PCNA) protein were detected in the liver cancer tissues by immunohistochemical method.
RESULTS: The Ki-67 protein expression was significantly lower in Groups C and D as compared with non-TACE group (31.35% ± 10.85% vs 44.43% ± 20.70%, 30.93% ± 18.10% vs 44.43% ± 20.70%, respectively, P < 0.05). The PCNA protein expression was significantly lower in Groups C and D as compared with non-TACE group (49.61% ± 15.11% vs 62.92% ± 17.21%, 41.16% ± 11.83% vs 62.92% ± 17.21%, respectively, P < 0.05). The Ki-67 protein expression was significantly higher in Group A as compared with non-TACE group (55.44% ± 13.72% vs 44.43% ± 20.70%, P < 0.05). The PCNA protein expression was significantly higher in Groups A and B as compared with non-TACE group (72.22% ± 8.71% vs 62.92% ± 17.21%, 69.91% ± 13.38% vs 62.92% ± 17.21%, respectively, P < 0.05).
CONCLUSION: Preoperative multi-material TACE suppresses the proliferation of HCC cells, while a single material embolization and chemotherapy alone enhance the proliferation of HCC cells.
- Citation: Xiao EH, Li JQ, Huang JF. Effect of preoperative transcatheter arterial chemoembolization on proliferation of hepatocellular carcinoma cells. World J Gastroenterol 2007; 13(33): 4509-4513
- URL: https://www.wjgnet.com/1007-9327/full/v13/i33/4509.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i33.4509
Hepatocellular carcinoma (HCC) is one of the most common malignancies. Curative local resection is recognized as a safe and effective method for patients with HCC[1]. Unfortunately, only a minority of patients currently diagnosed with HCC may benefit from this radical option. Transcatheter arterial chemoembolization (TACE) has become one of the most popular and effective palliative methods for patients with HCC. Various mixtures of anticancer drugs, iodized oil and gelatin sponge have been used as TACE agents. There have been a few reports on comparison of the efficacy of different TACE regimens on patients with HCC[2].
Proliferating cell nuclear antigen (PCNA) is an auxiliary factor in DNA polymerase, and is expressed in the nuclei, particularly in the late G1 and S phases. Ki-67 is expressed throughout the cell cycle (late G1, S, G2, and M phases) of proliferating cells, but is absent in quiescent (G0) cells. Therefore, PCNA and Ki-67 are believed to be useful markers for proliferative activity[3]. Our previous study showed that expression of p53 can enhance expression of PCNA and Ki-67 after TACE[4].
As far as we are aware, the effects of different TACE regimens on proliferation of HCC cells have not been investigated previously. In particular, it is unclear whether TACE can enhance or suppress proliferation of HCC cells by modulating the expressions of PCNA and Ki-67 proteins. In the present study, we examined the effects of the four main types of TACE used clinically (pure intra-arterial chemotherapy; chemotherapy plus iodized oil; chemotherapy plus iodized oil plus gelatin- sponge; chemotherapy plus iodized oil plus alcohol plus gelatin-sponge) on proliferation of HCC cells in vivo.
From Feb 1992 to Feb 2001, a total of 136 patients with HCC were referred to our hospital for surgery. There were 122 men and 14 women with mean age of 45 (ranged from 20 to 70) years. A diagnosis of HCC was obtained for all patients by preoperative ultrasound (US) or/and computed tomography (CT) or/and magnetic resonance image (MRI) or/and digital subtraction angiography (DSA) and plasma AFP levels and confirmed by pathological biopsy.
The patients were divided into two groups according to treatment manners. In the TACE group, 79 patients underwent 1-5 courses of chemoembolization prior to liver resection. In the control group, 57 patients received initial liver resection without preoperative TACE. The extent of liver resection was carried out based on the location of tumor, the severity of concomitant liver cirrhosis and preoperative liver reserve function.
By Seldinger’s technique, indirect portal-veinography through the superior mesenteric artery was firstly performed to observe portal vein flow, thrombus, mislocalized tumor-feeding artery. Then a catheter was inserted selectively and superselectively into the right or left hepatic artery or the tumor-feeding artery. The patients in TACE group were divided into four subgroups: one to four courses of only infusion of chemotherapeutic agents, including 5-fluorouracil (5-FU) 1000 mg (NanTong Pharmaceutical Factory, China), mitomycin-c (MMC) 10 mg (Kyowa Hakko Kogyo Co. Ltd., Japan), carboplatin 300 mg (QiLu Pharmaceutical Factory, China), or epirubicin (E-ADM) 60 mg (Zhejiang HiSun Pharmaceutical Co. Ltd., China), were performed in 11 patients (Group A); one to five courses of first infusion of the same chemotherapeutic agents as group A, then embolization with mixture composed by iodized oil (Lipiodol, Guerbet, France ) 5-20 mL according to the tumor size and E-ADM were performed in 33 patients (Group B); one to three courses of chemotherapy combined with iodized oil, the same as group B, plus adequate gelatin sponge particle embolization were performed in 23 patients (Group C); one to three courses of chemotherapy combined with iodized oil, ethanol and gelatin-sponge, that is, firstly, the same chemotherapy as group A, secondly, embolization with mixture composed of iodized oil 5-20 mL and waterless ethanol 1-5 mL (two ratio 4:1), finally, embolization with adequate gelatin sponge particle, were performed in 12 patients (Group D). Of them, 50 patients underwent one course of TACE; 19 patients underwent two courses of TACE; 10 patients underwent three or more courses of TACE during an interval of 52.8 ± 12.2 d (mean ± SD). Of them, 25 patients had ≤ 1 mo interval; 29 patients had ≤ 2 mo interval; 16 patients had ≤ 3 mo interval; and 9 patients had > 3 mo interval.
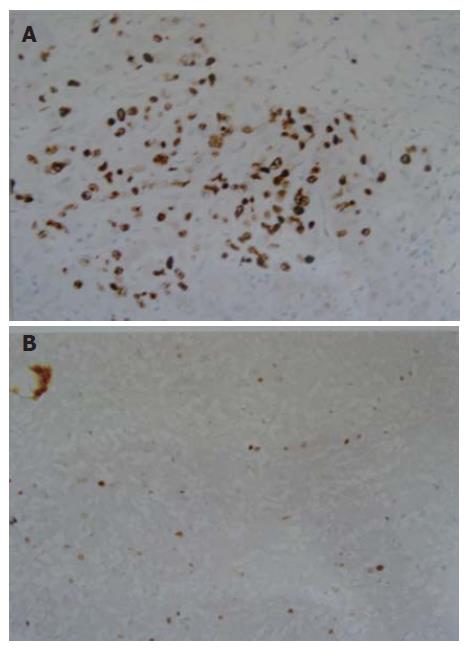
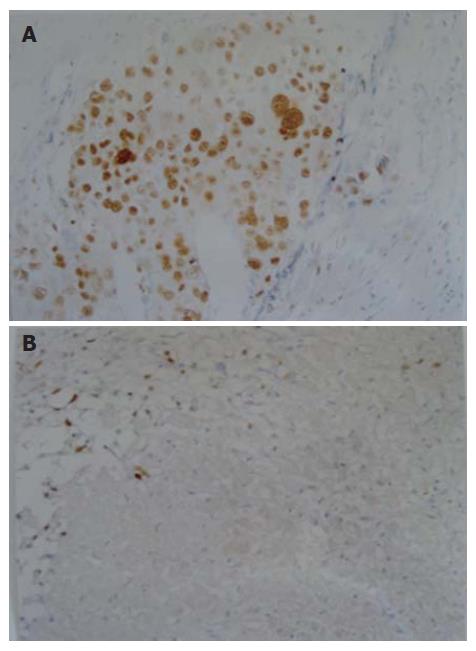
The formalin-fixed, paraffin-embedded specimens were examined immunohistochemically using anti-Ki-67 monoclonal antibody M7187 (1:50 dilution) and anti-PCNA monoclonal antibody M0879 (1:200 dilution) (LSAB kit Dako). Positive controls were normal lymph nodes. Negative controls were generated by substituting for the primary antibody with a non-specific IgG (normal rabbit IgG) and tris-buffered saline. Ki-67- and PCNA-positive cells showed brown-yellow staining in the nuclei of cancer cells (Figures 1 and 2). Rate of positive immunostaining for Ki-67 or PCNA was calculated as the ratio of the number of positively stained tumor cells to the total number of tumor cells counted per section. All slides were reviewed and scored in a blind fashion by two observers without knowledge of the corresponding clinical data. A few cases with discrepant scoring were revaluated jointly on a second occasion, and an agreement was reached.
Data were expressed as mean ± SD and analyzed by means of SPSS 10.0 software package (SPSS, Chicago, IL, USA, 1999) using Student’s t test, Crosstabs (Chi-square and Fisher exact probability test) and K Independent Samples, when appropriate A P value < 0.05 was considered statistically significant.
Ki-67 and PCNA protein expressions of HCC cells, respectively, were 44.43% ± 20.70% and 62.92% ± 17.21% in non-TACE group, 55.44% ± 13.72% and 72.22% ± 8.71% in Group A, 45.26% ± 14.97% and 69.91% ± 13.38% in Group B, 31.35% ± 10.85% and 49.61% ± 15.11% in Group C, and 30.93% ± 18.10% and 41.16% ± 11.83% in Group D. Ki-67 protein expression was significantly higher in Groups A and B as compared with Groups C and D, was lower in Groups C and D as compared with non-TACE group, and was higher in Group A as compared with non-TACE group (P < 0.05). PCNA protein expression was significantly higher in Groups A and B as compared with Groups C, D and non-TACE group, and was lower in Groups C and D as compared with non-TACE group (P < 0.05).
Ki-67 and PCNA protein expressions of HCC cells, respectively, were 44.43% ± 20.70% and 62.91% ± 17.21% in non-TACE group, 41.34% ± 16.69% and 62.00% ± 18.47% in one-course of TACE group, 39.24% ± 14.48% and 57.70% ± 15.54% in two-courses of TACE group, and 38.33% ± 20.90% and 53.97% ± 18.13% in three- or four- or five-courses of TACE group, indicating that Ki-67 and PCNA protein expressions were insignificantly decreased as the courses of TACE increased.
Ki-67 and PCNA protein expressions of HCC cells, respectively, were 44.43% ± 20.70%, 62.91% ± 17.21% in non-TACE group, 35.42% ± 15.46% and 53.19% ± 18.28% in ≤ 1 mo interval of TACE group, 40.66% ± 18.53% and 58.09% ± 15.90% in 1-2 mo interval of TACE group, 42.22% ± 14.80% and 66.83% ± 17.93% in 2-3 mo interval of TACE group, and 50.62% ± 12.33% and 72.53% ± 12.93% in > 3 mo interval of TACE group. Comparison between groups indicated that the Ki-67 protein expression was significantly lower in “≤ 1 mo" interval group as compared with “> 3 mo” interval and non-TACE groups (P < 0.05), while the expression of PCNA protein was significantly lower in the “≤ 1 mo” and “1-2 mo” interval groups as compared with “> 3 mo” interval and non-TACE groups (P < 0.05).
HCC is one of the most common malignant neoplasms. The majority of the HCC patients are treated with palliative approaches to improve the respectability rate and prolong survival. TACE has been one of the most common and effective palliative approaches. The prognosis of patients treated with TACE depends not only on use of an effective TACE regimen but also on tumor factors[5].
To our knowledge, few data currently are available regarding the molecular mechanism of TACE treatment for patients with HCC, and the current study is the first report detailing the correlations between the expressions of Ki-67 and PCNA protein and different TACE regimens.
The two proliferative indices assessed in our study were Ki-67 and PCNA. Ki-67 presents throughout the cell cycle (late G1, S, G2, and M phases) of proliferating cells, but is absent in quiescent (G0) cells[6]. PCNA is a non-histone nuclear protein of 36 kDa, an auxiliary pro-DNA polymerase δ that plays a major role in synthesizing DNA, and is believed to be expressed in the nuclei, particularly in the late G1 and S phases[6]. Univariate and multivariate analyses showed that the high labeling index of PCNA resulted in high tumor recurrence risk, more aggressive growth and poor survival[7-9].
The current study demonstrated that the effects of TACE on proliferation of HCC cells depended on its regimens. We found that the mean percentage of Ki-67 and PCNA protein expression was in a decreasing order as follows: Groups A and B > non-TACE group > Group C and D, which suggested that TACE using iodized oil, gelatin sponge particle and/or ethanol significantly inhibited proliferation of HCC cells, whereas TACE using iodized oil alone and chemotherapy alone increased proliferation of HCC cells, which is in agreement with our previous reports that TACE using iodized oil, gelatin sponge particle and/or ethanol significantly decreased proliferative index (PI) and S-phase fraction (SPF) of HCC cells[10]; alone iodized oil TACE and chemotherapy alone increased PI and SPF of HCC cells[10]; PCNA protein expression of HCC cells was significantly higher in the TACE group which mostly consisted of iodized oil and anticancer drugs[11], and multi-material TACE easily resulted in decreasing of HCC volume[12].
The best interval of treatment for repeated TACE or second stage resection is controversial. Hsu et al[13] considered 3 to 21 d interval was adequate to prevent the regrowth of residual tumor cells. Zhu et al[14] considered 1 to 3 mo interval was best for resectable tumors, and longer interval for unresectable tumors. Moreover, 1 to 2 mo interval by Liang et al[15], 2 to 3 mo interval by Lai et al[16], 3 mo interval by Zhang et al[17], 3 to 4 mo interval by Kenji et al[18], and > 3 mo interval by Teng et al[19] were proposed as the best interval. The above-mentioned data were based on the clinical and pathological data. In this study, we found that there were significantly lower Ki-67 and PCNA protein expressions in the “≤ 1 mo” and “> 1 and ≤ 2 mo” interval Groups as compared with “> 3 mo” interval Group (P < 0.05). In other word, the remaining cancer cells after TACE treatment had significantly lower proliferative activity at 1 to 2 mo interval than > 3 mo interval. According to this molecular and genetic study and previous clinical and pathological study, we considered the best interval of treatment for repeated TACE or second stage resection is between 2 and 3 mo.
This study demonstrated that Ki-67 and PCNA protein expressions decreased as the courses of TACE increased. Taken collectively, the present data together with our previous study[12] and findings by Spreafico et al[20] and Lai et al[16] that tumor necrosis, shrinkage of the tumor mass, proliferation and encapsulation of perimass fibrous tissue were closely related to the courses of TACE and findings by Zhang et al[21] that the survival in patients with multi-times TACE was better than those with single one, suggest that TACE could be performed multi-times, provided the patients’ condition is preferable. But the liver cirrhosis rate after TACE treatment had significant correlation with the courses of treatment[22]. The selective and superselective catheterization is the best way to avoid damaging the normal liver tissue.
In conclusion, the present study demonstrates that the proliferative activity of residual HCC cells after being treated by TACE using iodized oil, gelatin sponge, and/or ethanol is significantly decreased as compared with TACE using iodized oil alone or pure intra-artery chemotherapy. The effect of TACE on proliferation of HCC cells has negative correlation with number of course of TACE and positive correlation with the interval of TACE.
Transcatheter arterial chemoembolization (TACE) has become one of the most popular and effective palliative methods for patients with HCC. Various mixtures of anticancer drugs, iodized oil and gelatin sponge, have been used as TACE agents. There have been a few reports on comparison of the efficacy of different TACE regimens on patients with Hepatocellular carcinoma (HCC).
The effects of different TACE regimens on proliferation of HCC cells had not been investigated previously.
In the present study, the effects of the four main types of TACE used clinically on proliferation of HCC cells in vivo have been examined.
Best mixtures and methods could be used for TACE.
TACE: Transcatheter arterial chemoembolization.
As mentioned TACE is one of the most popular and effective palliative method for patients with HCC. Various mixtures and methods had been used for TACE. In this study, the effectiveness of the TACE methods was compared. It is an interesting study.
S- Editor Zhu LH L- Editor Kumar M E- Editor Wang HF
| 1. | Cai J, Hu J, Che X, Zhao J, Bi X, Shao Y. Prognosis of primary liver carcinoma treated with local resection. Chin Med J (Engl). 2003;116:187-190. [PubMed] |
| 2. | Ueno K, Miyazono N, Inoue H, Nishida H, Kanetsuki I, Nakajo M. Transcatheter arterial chemoembolization therapy using iodized oil for patients with unresectable hepatocellular carcinoma: evaluation of three kinds of regimens and analysis of prognostic factors. Cancer. 2000;88:1574-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 3. | Perry A, Jenkins RB, O'Fallon JR, Schaefer PL, Kimmel DW, Mahoney MR, Scheithauer BW, Smith SM, Hill EM, Sebo TJ. Clinicopathologic study of 85 similarly treated patients with anaplastic astrocytic tumors. An analysis of DNA content (ploidy), cellular proliferation, and p53 expression. Cancer. 1999;86:672-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Xiao EH, Li JQ, Huang JF. Effects of p53 on apoptosis and proliferation of hepatocellular carcinoma cells treated with transcatheter arterial chemoembolization. World J Gastroenterol. 2004;10:190-194. [PubMed] |
| 5. | Rose DM, Chapman WC, Brockenbrough AT, Wright JK, Rose AT, Meranze S, Mazer M, Blair T, Blanke CD, Debelak JP. Transcatheter arterial chemoembolization as primary treatment for hepatocellular carcinoma. Am J Surg. 1999;177:405-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Igarashi N, Takahashi M, Ohkubo H, Omata K, Iida R, Fujimoto S. Predictive value of Ki-67, p53 protein, and DNA content in the diagnosis of gastric carcinoma. Cancer. 1999;86:1449-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Fukuse T, Hirata T, Naiki H, Hitomi S, Wada H. Expression of proliferating cell nuclear antigen and CD44 variant isoforms in the primary and metastatic sites of nonsmall cell lung carcinoma with intrapulmonary metastases. Cancer. 1999;86:1174-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Kitamoto M, Nakanishi T, Kira S, Kawaguchi M, Nakashio R, Suemori S, Kajiyama G, Asahara T, Dohi K. The assessment of proliferating cell nuclear antigen immunohistochemical staining in small hepatocellular carcinoma and its relationship to histologic characteristics and prognosis. Cancer. 1993;72:1859-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Li JQ, Zhang CQ, Zhang YQ. Immunohistochemical study of PCNA and p53 in primary liver cancer: An implication for prognosis and treatment. J Exp Clin Cancer Res. 1996;15:77-82. |
| 10. | Xiao EH, HU GD, Li JQ, Zhang YQ, Chen MS. The effect of different chemoembolization methods on PI, SPF and DI in different histopathological types of hepatocellular carcinoma. Linchuang Fangshexue Zazhi. 2001;20:624-627. |
| 11. | Huang J, He X, Lin X, Zhang C, Li J. Effect of preoperative transcatheter arterial chemoembolization on tumor cell activity in hepatocellular carcinoma. Chin Med J (Engl). 2000;113:446-448. [PubMed] |
| 12. | Xiao EH, Hu GD, Li JQ, Zhang YQ, Chen MS, Guo YP, Lin XJ, Li SP, Li GH. The relationship between the tumor necrosis and transcatheter arterial chemoembolization methods for hepatocellular carcinoma. Linchuang Fangshexue Zazhi. 2000;19:513-515. |
| 13. | Hsu HC, Sheu JC, Lin YH, Chen DS, Lee CS, Hwang LY, Beasley RP. Prognostic histologic features of resected small hepatocellular carcinoma (HCC) in Taiwan. A comparison with resected large HCC. Cancer. 1985;56:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Zhu ZG, Cuo BH. Analyhuosis of efficacy of resection of liver cancer after transhepatic artery chemoembolization. Gandanyi Waike Zazhi. 1997;9:158-159. |
| 15. | Liang LJ, Lin HL, Huang JF. The clinical and pathological features of resected hepatocellular carcinoma after hepatic arterial chemoembolization. Aizheng Zazhi. 1993;12:148-150. |
| 16. | Lai RQ, Hao CZ, Wang JX. Pathomorphological study of resected hepatocellular carcinoma after transcatheter hepatic arterial chemoembolization. Zhonghua Binglixue Zazhi. 1993;22:19-21. |
| 17. | Zhang BH, Quan GX, Wu MC. Selection of surgical time for resectableliver cancer after transcatheter hepatic artery embolization. Gandanyi Waike Zazhi. 1997;9:153-155. |
| 18. | Ikeda K, Kumada H, Saitoh S, Arase Y, Chayama K. Effect of repeated transcatheter arterial embolization on the survival time in patients with hepatocellular carcinoma. An analysis by the Cox proportional hazard model. Cancer. 1991;68:2150-2154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Teng GJ, He SC, Guo JH. Resection of hepatocellular carcinoma folloeing transcatheter hepatic artery embolization. Zhonghua Fangshexue Zazhi. 1994;28:597-600. |
| 20. | Spreafico C, Marchianò A, Regalia E, Frigerio LF, Garbagnati F, Andreola S, Milella M, Lanocita R, Mazzaferro V. Chemoembolization of hepatocellular carcinoma in patients who undergo liver transplantation. Radiology. 1994;192:687-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Zhang Z, Wu M, Liu Q. The effect of preoperative transcatheter hepatic arterial chemoembolization on disease-free survival after hepatectomy for hepatocellular carcinoma. Zhonghua ZhongLiu ZaZhi. 1999;21:214-216. [PubMed] |
| 22. | Xiao EH, Hu GD, Li JQ, Zhang YQ, Chen MS, Guo YP, Lin XJ, Li SP, Li GH. The relationship between the tumor tissue reaction and transcatheter arterial chemoembolization methods for hepatocellular carcinoma. Shiyong Fangshexue Zazhi. 2001;17:324-326. |