Published online Aug 28, 2007. doi: 10.3748/wjg.v13.i32.4355
Revised: March 23, 2007
Accepted: March 31, 2007
Published online: August 28, 2007
AIM: To study the effect of moxibustion on Zusanli or Liangmeng point on gastric mucosa injury in stress-induced ulcer rats and its correlation with the expression of heat shock protein 70 (HSP70).
METHODS: Sixty healthy SD rats (30 males, 30 females) were divided into control group, injury model group, Zushanli point group, Liangmeng point group. Stress gastric ulcer model was induced by binding cold stress method. Gastric mucosa ulcer injury (UI) index was calculated by Guth method. Gastric mucosa blood flow (GMBF) was recorded with a biological signal analyzer. Protein content and gene expression in gastric mucosal HSP70 were detected by immunohistochemistry (IHC) and reverse transcription polymerase chain reaction (RT-PCR). Thiobarbital method was used to determine malondialdehyde (MDA) content. Gastric mucosal endothelin (ET) and prostaglandin E2 (PGE2) were analyzed by radioimmunoassay.
RESULTS: High gastric mucosal UI index, high HSP70 expression, low GMBF and PGF2, elevated MDA and ET were observed in gastric mucosa of rats subjected to cold stress. Moxibustion on Zusanli or Liangmeng point decreased rat gastric mucosal UI index, MDA and ET. Conversely, the expression of HSP70, GMBF, and PGE2 was elevated in gastric mucosa after pretreatment with moxibustion on Zusanli or Liangmeng point. The observed parameters were significantly different between Zusanli and Liangmeng points.
CONCLUSION: Pretreatment with moxibustion on Zusanli or Liangmeng point protects gastric mucosa against stress injury. This protection is associated with the higher expression of HSP70 mRNA and protein, leading to release of PGE2 and inhibition of MDA and ET, impairment of gastric mucosal index.
- Citation: Chang XR, Peng L, Yi SX, Peng Y, Yan J. Association of high expression in rat gastric mucosal heat shock protein 70 induced by moxibustion pretreatment with protection against stress injury. World J Gastroenterol 2007; 13(32): 4355-4359
- URL: https://www.wjgnet.com/1007-9327/full/v13/i32/4355.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i32.4355
Recent studies indicate that moxibustion can protect gastric mucosa against injury[1-3]. However, its exact mechanism remains unclear. Several strategies have been proposed recently. Heat shock protein (HSP), the cellular self-protective defense factor, is one of the hot spots. HSP70 is one of the important HSPs in gastric mucosal protection[4]. To explore the involvement of HSP70 in gastric mucosal protection, we studied the effects of moxibustion pretreatment on the expression of HSP70 mRNA expression and protein with the purpose of clarifying its endogenous protective mechanism. We believe that the experiment results provide a useful tool to induce HSPs via traditional Chinese medicine.
Sixty healthy SD rats (30 males, 30 females), weighing 200-250 g, were provided by College of Animal Sciences & Technology, Hunan Agriculture University. Endothelin (ET) and malondialdehyde (MDA) detection kits were provided by Radioimmunoassay Institute of Scientific and Technological Development Center of PLA General Hospital. Other regents were of analytical purity. Rabbit anti-rat HSP70 affinity-purified antibody and DEPC were purchased from Wuhan Boshide Company. SP detection kit and DAB yellowbrown color detection kit were purchased from Beijing Zhongshan Golden Bridge Biotech Limited Company. Trizol reagent kit was from Invitrogen (USA). AMV reverse transcription enzyme, RNasin, dNTPs, Taq DNA polymerase, 100 bp DNA ladder were from Promega (USA). PGE2 detection kit was from Institute of Blood, Suzhou University. Aizhu was purchased from Suzhu Oriental Aiyong Institute (“Shenjiu 300 Jiu”, Oriental type 1).
The patients were divided into control group (I), injury model group (II), Zushanli point group (III), Liangmeng point group (IV).
The control point[5] was located 1 cm from Liangmeng point and no specific point was located between knee joint and Zusanli.
Rats in groups I, II were not treated with moxibustion, whereas rats in groups III, IV were treated with moxibustion (4 times a day, 30 min each time, for 8 d) on the points located at one side. After the hair was removed, AiZhu was pasted on the points located on both sides (4 times a day, 30 min each time, for 8 d). Seven days after moxibustion pretreatment, acute stress gastric ulcer model was established as previously described[6]. Rats in groups II, III, IV were treated for six days with no access to food and water for 24 h on the last day. These rats were submerged into 20°C water at the level of bottom of breastbone and released 10 h after submersion.
All animals were anesthetized with 10% urethane intraperitoneal injection 24 h after establishment of stress model. Five rats in each group were used for immunohistochemical analysis. The pylorus portion of stomach was ligated and 3 mL of 4% formaldehyde was infused into the stomach from esophagus. The cardiac portion of stomach was ligated after the needle was pulled out. The esophagus and duodenum were cut off from the ligated sites. The stomach was extracted and dissected along the gastric greater curvature after 10 min. The stomach content was washed out with normal saline and fixed with 4% formaldehyde for 24 h. Paraffin sections were prepared.
The other rats were anesthetized and dissected as described above. A small piece of mucosa on the gastric sinas portion was prepared (about 50 mg), washed three times with 0.1% DEPC solution, and stored in liquid nitrogen. The other portions of gastric mucosa were washed with ice cold normal saline. Gastric mucosa ulcer injury (UI) index was calculated and the gastric mucosa was weighed with an analytical balance. A certain amount of normal saline was added to the 1.5 mL/400 mg concentration. Gastric mucosal tissue homogenate was prepared with a glass-glass homogenizer and centrifuged at 35 000 r/min for 15 min at 4°C. Supernatant was extracted and stored at -20°C for assay.
According to GUTH method, the summary of gastric mucosal impairment site length was calculated as UI index and expressed as millimeter. The standard score was: 1 point: < or = 1 mm; 2 points: 1 mm < damage < or = 2 mm; 3 points: 2 mm < damage < or = 3 mm; 4 points: 3 mm < damage < or = 4 mm; 5 points: > 4 mm. The UI index should be doubled if the width of injury was more than 2 mm.
Paraffin sections (4 μm thick) were prepared. Tissue sections were deparaffinized and hydrated in xylenes and graded alcohol. The sections were incubated with primary anti-HSP70 diluted in buffer. PBS was used as negative control. The positive results were analyzed with the MIAS medical imaging analytical system. Five scopes on each section were quantified under microscope. The average density of each section was calculated.
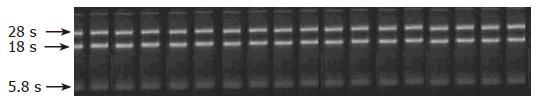
RT-PCT technique was used to analyze the expression of HSP70 mRNA in rat gastric mucosa. Total RNA was extracted from rat gastric mucosa with TRIzol method. Ten μL of RNA sample was taken to analyze the purity and completeness of RNA (Figure 1). Three mol/L and 1/10 volume of NaAc (pH 5.2) and ethanol were dehydrated 3 times and stored at -20°C or -70°C.
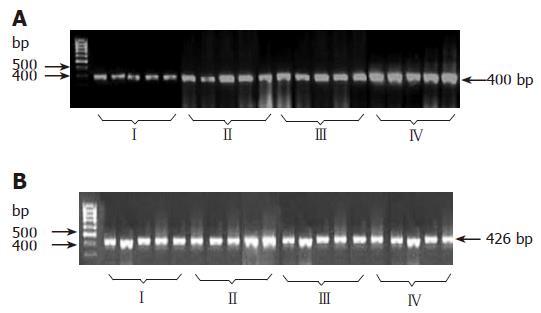
Three μg of total RNA was used for RT-PCR (20 μL total volume). Three μg RNA, 0.5 μg oligo (dT), 18 primers, 20 U RNasin, 10 mmol/L dNTPs, 5 × RT buffer, and 10 U AMV reverse transcriptase were mixed and heated at 42°C for 1 h. The cDNA product was used as PCR reaction source of gene for analysis. The primeres of rat HSP70 were synthesized by Invitrogen Biotech Company. The routine PCR reaction (25 μL total volume) included: 2.5 μL 10 × PCR buffer, 1.5 mmol/L MgCl2, 200 μmol/L dNTPs, 2 μL cDNA, 0.1 μmol/L specific primers, 2 U Taq DNA polymer, and paraffin oil for cover. The HSP70 cDNA in PCR products was 268 bp. The reaction conditions were set at 94°C for 2 min, at 94°C for 30 s, at 56°C for 30 s, at 72°C for 30 s, for 35 cycles. The cDNA in PCR products was 426 bp. The reaction conditions were set at 4°C for 2 min, at 94°C for 30 s, at 56°C for 30 s, at 72°C 30 s, for 25 cycles. Ten μL of HSP70 cDNA and 10 μL of GAPDH were loaded onto 1.5 % agarose gel for electrophoresis. The absorbance (A) of gel electrophoresis products was scanned and read. The ratio of HSP70 cDNA/GAPDH cDNA was used for observed parameters.
Rats were anesthetized and operated along the mid-line of abdomen to expose the stomach. Laser Doppler blood flow meter and miniature surface probes (Biopac Inc. USA) were used to record the blood flow. The acquired signal was converted to blood perfusion unit (BPU) and input into a computer. The curve was recorded with Acqknowledge v3.5 software. A 2 cm opening cut was made at the greater curvature of stomach. Laser probes were inserted into the sinus, bottom, greater and lesser curvature of stomach. The data were obtained only after the measurement curve on the display became stable. Measurement was performed three times (15 s for each time) on each point for every rat. The average value was designated as the 10 s stabling curve. The final observed parameters were obtained from the average value of four different points.
Radioimmunoassay was performed to detect ET and PGE2 in gastric mucosa according to the instructions of ready-use reagent from the Radioimmunoassay Institute of Scientific and Technological Development Center of PLA General Hospital, and Institute of Blood, Suzhou University, China. Thiobarbital was used to determine malondialdehyde (MDA) following the instructions from Radioimmunoassay Institute of Scientific and Technological Development Center of PLA General Hospital.
The data were analyzed with SPSS 11.5 software and one way analysis of variance (ANOVA), and expressed as mean ± SD. P < 0.05 was considered statistically significant.
As shown in Table 1, UI index was significantly lower in group III than in groups II, IV (P < 0.01) and no difference was observed between groups III andI (P > 0.05). UI Index was higher in group II than in groupI(P < 0.01). ET was markedly lower in group III than in group II (P < 0.05) and there was no difference between groups III andI (P > 0.05). ET was elevated in group II after stress stimulation (P < 0.05 vs groupI). PGE2 was significantly increased in group III after moxibustion (P < 0.05 vs group II). No difference was observed between groups III andI(P > 0.05). PGE2 was lower in group II than in groupI(P < 0.05).
As shown in Table 2, MDA was significantly lower in group III than in group II (P < 0.05) and no difference was observed between groups III andI(P > 0.05). MDA was higher in group II than in groupI(P < 0.05). Moxibustion also improved GMBF (P < 0.05 vs group II) and no difference was observed between groups III andI. Cold stress stimulation alone attenuated GMBF (P < 0.01 vs groupI).
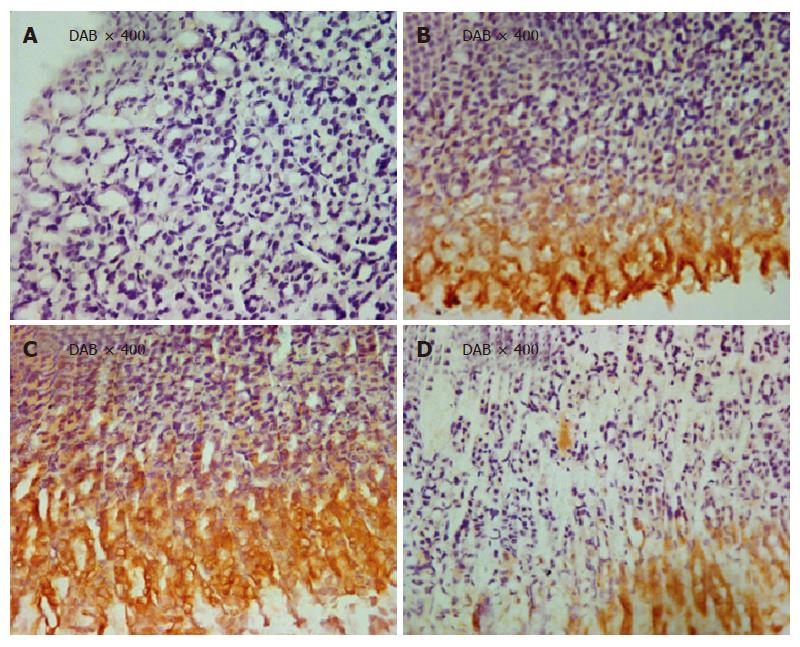
The immunohistochemical results indicated that HSP70 positive portion (yellow-brown color) was detected in cytoplasm, mostly in sinus of stomach. Moxibustion on points significantly enhanced the expression of HSP70 (P < 0.01 vs group II; P < 0.05 vs group IV). No difference was observed between groups III andI) (P > 0.05). Interestingly, HSP70 expression was markedly higher in group II than in groupI(P < 0.05) (Table 2).
As shown in Figure 2, HSP70 gene expression was significantly elevated in group III (P < 0.01 vs groupsI, II, and IV). Correspondingly, HSP70 gene expression also enhanced in group II (P < 0.01 vs groupI).
A panel of representative photomicrographs showed HSP70 staining in gastric mucosa from rats in different groups (Figure 3 A-D).
HSPs are a group of highly conserved stress proteins and play an important role in maintaining body self-stability. The main functions of HSPs are to promote cellular tolerance against stress factors, to maintain cellular normal physiological function, and to increase defense against and adaptation of cells to deadly stimulation[7]. Over-expression of HSP70 has been observed in rats with acute and chronic gastric ulcer, chronic atrophic gastritis, and in patients with gastric cancer. The most leading over-expression of HSP70 has been found in apparent pathological portion[8-10]. Over-expression of HSP70 promotes ulcer healing by increasing gastric mucosal blood flow, and cell multiplication[11-13]. Expression of HSP70 can also be induced with heat pretreatment, long term stimulation with low ethanol, and long term administration of aspirin. The expression of HSP70 is associated with adaptive cellular protection-tolerances to stimulation of hyperthermia, high concentration of alcohol, and high doses of aspirin[14-16]. Moxibustion acts as a kind of physiological mild stimulator to induce HSP70 production. This effect connects its therapeutic application to immunogenic property for activation of immunological system[17,18]. Our study indicates that cold stress stimulation could increase HSP70 protein and gene expressions in rat gastric mucosa (P < 0.05 vs control group), suggesting that pretreatment with moxibustion can significantly enhance the expressions of HSP70 protein (P < 0.01 vs stress model group).
Stress gastric injury is attributed to some factors that decrease the function of gastric mucosa defense system and relatively increase the function of impairment system. Endogenous prostaglandins are a kind of protective substances in gastric mucosa and can increase secretion of gastric media and biocarbonate, promoting surface hydrophobity and gastric mucosal blood flow. Conversely, endothelin, a potent vasoconstrictor, constricts gastric mucosal blood flow leading to dysfunction of gastric circulation and damage to gastric mucosa[19]. Our results showed higher PGE2 and lower ET in group III (moxibustion, P < 0.05), which were related to the improvement of gastric mucosal damage (P < 0.01). Oxidative stress plays an important role in stress-induced gastric mucosal injury and involves cellular apoptosis. It was reported that MDA increases 3 h after stress and reaches its peak within 6-12 h[20]. Free radical generation increases after long time exposure to stress. The free radical scavenger system is insufficient to remove the newly generated free radicals and leads to damage to gastric mucosa[21]. Electro-acupunture on Zusanli point decreases plasma MDA and improves oxygen free radical metabolism. These effects are related to its protective effects against stress[22]. In our study, gastric mucosal MDA was higher in group II than in groupsIand III (P < 0.05), indicating that moxibustion decreases gastric mucosal MDA and protects gastric mucosa against oxidative injury.
Channel connection with Zang and Fu organs (viscera) is one of the important theories in traditional Chinese medicine. “Twelve channels connect with arms and body surface outwardly and associate with Zang and Fu organs inwardly”. This theory has been described in detail in <<LingSu•HaiLun, miractous pivot, name of a book>>. Foot Yangming Wei Channel is a channel full of Qi and blood, and participates in the circulation of 14 channels and provides the sources for Qi and blood. It is one of the essential elements of Channel theory. Stomach (Wei) is the “field of water and food” and converts ingestant into nutrients and blood. Spleen and stomach (PiWei) are the essential parts for growth and development. Zusanli point, a co-point in bottom of stomach (Wei) and Wei Channel, is a first selection point for stomach disease. Liangmeng point is another point in foot Zusanli stomach channel. The present study indicated that moxibustion pretreatment on Zusanli and Liangmeng points increased HSP70 protein and gene expressions and PGE2 in gastric mucosa (P < 0.05 or 0.01 vs moxibustion pretreatment on no specific point). Moxibustion reduced damage to gastric mucosa (P < 0.01) and the enhanced MDA induced by gastric mucosal stress and exerted antioxidation, suggesting that Zusanli and Liangmeng points protect gastric mucosa against stress injury by a point-specific mechanism. These results provide strong evidence for the treatment of digestive illness with acupuncture method.
Moxibustion can prevent and cure diseases through mild warm stimulation and point-channel action. Modern clinical and experimental studies have confirmed that moxibustion has analgesic effects and improves blood circulation. Metabolism dysfunction, activity of Zang and Fu organs, and immunological function can also be improved with moxibustion[22].
In summary, moxibustion protects gastric mucosa against stress injury, which is related to the induction of HSP70 protein and mRNA expression. These results provide an alternative pathway to trigger endogenous protective substances with acupuncture. Effective induction of HSPs with means of traditional Chinese medicine is a useful tool to provoke endogenous defense system for prevention and treatment of diseases. However, whether moxibustion-induced HSP70 expression can protect other tissues or organs against stress injury remains to be further studied.
S- Editor Zhu LH L- Editor Wang XL E- Editor Li JL
| 1. | Hu G, Chen H, Hou Y, He J, Cheng Z, Wang R. A study on the clinical effect and immunological mechanism in the treatment of Hashimoto's thyroiditis by moxibustion. J Tradit Chin Med. 1993;13:14-18. [PubMed] |
| 2. | Waldum HL, Mårvik R, Grønbech JE, Sandvik AK, Aase S. Oxyntic lesions may be provoked in the rat both by the process of acid secretion and also by gastric acidity. Aliment Pharmacol Ther. 2000;14:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Liu J, Yu RC, Tang WJ. Influence of combined therapy of guben yiliu III, moxibustion and chemotherapy on immune function and blood coagulation mechanism in patients with mid-late stage malignant tumor. Zhongguo ZhongXiYiJieHe Za Zhi. 2002;22:104-106. [PubMed] |
| 4. | Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063-1081. [PubMed] |
| 5. | Li ZR. Experimental Acupuncture. Beijing: Chinese Medicine Press 2003; 327-329. |
| 6. | Kwiecień S, Brzozowski T, Konturek SJ. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J Physiol Pharmacol. 2002;53:39-50. [PubMed] |
| 7. | Takumida M, Anniko M. Heat shock protein 70 delays gentamicin-induced vestibular hair cell death. Acta Otolaryngol. 2005;125:23-28. [PubMed] |
| 8. | Shichijo K, Ihara M, Matsuu M, Ito M, Okumura Y, Sekine I. Overexpression of heat shock protein 70 in stomach of stress-induced gastric ulcer-resistant rats. Dig Dis Sci. 2003;48:340-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Shen X. Effects of cyclooxygenase-2 on formation and healing of acetic acid-induced gastric ulcer in rats. ZhonghuaYiXue ZaZhi. 2001;81:1380-1383. [PubMed] |
| 10. | Svestka T, Krechler T, Brůha R, Jablonská M. [Protective mechanism of the gastric mucosa]. Cas Lek Cesk. 2005;144 Suppl 1:40-43. [PubMed] |
| 11. | Tsukimi Y, Nakai H, Itoh S, Amagase K, Okabe S. Involvement of heat shock proteins in the healing of acetic acid-induced gastric ulcers in rats. J Physiol Pharmacol. 2001;52:391-406. [PubMed] |
| 12. | Isomoto H, Oka M, Yano Y, Kanazawa Y, Soda H, Terada R, Yasutake T, Nakayama T, Shikuwa S, Takeshima F. Expression of heat shock protein (Hsp) 70 and Hsp 40 in gastric cancer. Cancer Lett. 2003;198:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Al Moutaery AR. Protective effect of ketoconazole against experimentally induced gastric ulcers in rats. Res Commun Mol Pathol Pharmacol. 2003;113-114:5-23. [PubMed] |
| 14. | Tsukimi Y, Okabe S. Recent advances in gastrointestinal pathophysiology: role of heat shock proteins in mucosal defense and ulcer healing. Biol Pharm Bull. 2001;24:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Jin M, Otaka M, Okuyama A, Itoh S, Otani S, Odashima M, Iwabuchi A, Konishi N, Wada I, Pacheco I. Association of 72-kDa heat shock protein expression with adaptation to aspirin in rat gastric mucosa. Dig Dis Sci. 1999;44:1401-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Nikaido H, Tsunoda H, Nishimura Y, Kirino T, Tanaka T. Potential role for heat shock protein 72 in antagonizing cerebral vasospasm after rat subarachnoid hemorrhage. Circulation. 2004;110:1839-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Kobayashi K. Induction of heat-shock protein (hsp) by moxibustion. Am J Chin Med. 1995;23:327-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Li ZK. Gastric mucosa injury and protection- basic and clinical study. Shanghai: Scientific and Technological Publishing House 2004; 255-269. |
| 19. | Chung H, Kim E, Lee DH, Seo S, Ju S, Lee D, Kim H, Park S. Ghrelin inhibits apoptosis in hypothalamic neuronal cells during oxygen-glucose deprivation. Endocrinology. 2007;148:148-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 20. | Kwiecień S, Brzozowski T, Konturek PC, Pawlik MW, Pawlik WW, Kwiecień N, Konturek SJ. Gastroprotection by pentoxyfilline against stress-induced gastric damage. Role of lipid peroxidation, antioxidizing enzymes and proinflammatory cytokines. J Physiol Pharmacol. 2004;55:337-355. [PubMed] |
| 21. | Katsuyama M, Fan C, Arakawa N, Nishinaka T, Miyagishi M, Taira K, Yabe-Nishimura C. Essential role of ATF-1 in induction of NOX1, a catalytic subunit of NADPH oxidase: involvement of mitochondrial respiratory chain. Biochem J. 2005;386:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Meng Z, Zou Y, Luo E, Zou L. The development of clinical application of the rejuvenator and a study of its mechanism for the treatment of functional erectile dysfunction. ShengWu Y Xue Gon ChengXue ZaZhi. 2001;18:658-660. [PubMed] |