INTRODUCTION
Autoimmune hepatitis (AIH) is a predominant periportal hepatitis with hypergammaglobulinemia and tissue autoantibodies, which is responsive to immunosuppressive therapy in most cases[1]. Experimental autoimmune hepatitis (EAH) shares several features with human AIH periportal hepatitis[2], such as lymphocytic infiltrates[3,4], T cell reactivity to liver antigens[3], autoantibody production[5], and response to immunosuppressive therapy[6]. Experiments on EAH in inbred mice could give valuable information on effector cells and regulatory phenomenon in liver-specific immune reactions, while they could not induce a chronic relapsing AIH[7].
p38 MAPK, a stress-activated serine/thionine protein kinase that belongs to the the mitogen-activated protein kinase (MAPK) superfamily, is expressed ubiquitously, with high levels in the liver, spleen, thyroid, placenta, bone marrow, and leukocytes[8]. p38 MAPK activation is involved in the pathogenesis of human autoimmune diseases, including sialoadenitis of Sjögren syndrome[9], rheumatoid arthritis[10], inflammatory bowel disease[11] and autoimmune renal injury in systemic lupus erythematosus[12]. Activation of p38 MAPK may contribute to the pathogenesis of autoimmune diseases via the activation of the signal transduction and expression of cytokines and chemokines[12]. In liver, there is evidence that highlights a central role of MAPK family in several effects of ethanol[13] and hepatitis viruses such as hepatitis B, C and E viruses[14]. Recently, Tsutsumi et al[15] showed that HCV core protein activates ERK and p38 MAPK cooperatively with ethanol and modulates the expression of several genes related to cell transformation, cell cycle, and antioxidants in a mouse model of HCV-associated hepatocyte carcinoma. Interestingly, Gilbert et al[16] reported that trichloroacetaldehyde, one of the major metabolites of trichloroethylene, promotes T-cell activation via stimulation of MAPK pathway. It has been shown previously by the same group that MRL+/+ mice exposed to trichloroethylene in their drinking water develop lupus-like symptoms and AIH[17].
In the present study, we investigated the activation of p38 MAPK signaling pathway as well as nuclear factor-κB in the liver of a murine experimental model of autoimmune hepatitis induced by hepatic S100 antigen in complete Freund’s adjuvant. We further examined the protective effects of SB203580, a specific inhibitor of p38 MAPK, on the expression of proinflammatory cytokines, such as IFN-γ, IL-12, IL-1β, TNF-α in this model.
MATERIALS AND METHODS
Antigen preparation and induction of EAH
Fresh liver antigens from syngeneic animals were prepared after perfusion of livers with phosphate-buffered saline (PBS) as described previously[3,6]. Briefly, livers were homogenized on ice. After 10 min of centrifugation at 150 ×g, supernatant was collected and re-centrifuged for 1 h at 100 000 ×g. The remaining supernatant was used for immunization (called S100).
EAH was induced as described previously[3,6] with a minor modification. Freshly prepared syngeneic S-100 antigen at a dose of 0.5-2 mg/mL in 0.5 mL PBS emulsified in an equal volume of complete Freund’s adjuvant (CFA, Sigma-Aldrich, St. Louis, MO, USA) was injected intraperitoneally. All visible portal tracts of a liver section were evaluated by a pathologist in a blinded fashion and graded as 0: normal histomorphology, 1: minor inflammatory infiltrates with occasional liver cell necrosis, 2: moderate liver damage with inflammatory infiltrates and focal necroses, and 3: extensive infiltrates in portal tracts and lobules accompanied with diffusely distributed liver cell necroses. At least two separate sections were assessed per liver[2,3]. Serum alanine aminotransferase (ALT) levels were measured with an autoanalyzer.
Adult (age 5-7 wk) male wild type C57BL-6 mice (5-7 wk old) were obtained from the Central Animal Experimentation Facility of Renji Hospital, Shanghai Second Medical University (SSMU). All mice were maintained in a temperature- and light-controlled facility, and had free access to water and pellet chow. We observed the expression of phosphorylated p38 MAPK and NF-κB in mouse liver taken on d 1, 3, 7, 14, 21, 28 after immunization. Three mice were sacrificed at each time point. For p38 MAPK inhibition experiments, SB 203580 (Calbiochem, Shanghai, China, 10 μmol/kg) in 3% of DMSO was injected intraperitoneally (i.p.) daily into the mice. The mice were then killed after 14 d to obtain serum and liver tissue. The control mice were injected with vehicle (3% DMSO) or PBS. All animal experiments fulfilled the SSMU criteria for humane treatment of laboratory animals.
Western blot analysis
Cytoplasmic and nuclear protein extracts from liver were prepared with the NE-PER® nuclear and cytoplasmic extraction reagent kit (Pierce, Beijing, China) according to the manufacturer’s instructions. To minimize proteolysis, all buffers contained protease inhibitor cocktail (Roche, Shanghai, China). Protein concentration was determined by Bradford assay (Bio-Rad, Hercules, CA, USA). Equal amounts of proteins (30-50 μg) were separated by SDS-PAGE (10% running, 4% stacking gel) and transferred to PVDF membranes (Millipore, Bedford, MA, USA) in 192 mmol/L glycine, 25 mmol/L Tris, and 10% methanol. Nonspecific sites on membranes were blocked with 5% BSA in TBST overnight at 4°C. The membrane was incubated overnight at 4°C with a rabbit polyclonal antibody to phospho-p38 (Cell Signaling Technology Inc, MA, USA) at a dilution of 1:1000 followed by horseradish peroxidase-coupled donkey anti-rabbit Ig antibody (Santa Cruz Biotechnology, Santa Cruz, California, USA) at a dilution of 1:3000. Signals were detected using ECL Western blotting detection reagents (Amersham Bioscience, Castle Hill, Australia). The membrane was then stripped using Immuno pure IgG elution buffer (Pierce, Bejing, China) and reprobed with an antibody specific for total p38 (Cell Signaling Technology, Hong Kong, China). Signal intensity was quantified using a gel documentation system (Fluor-S-MultiImager and Quantity one software version 4.1, Bio-Rad). The level of total p38 served as an internal standard for protein loading and transfer.
Immunoprecipitate kinase activity assay for p38 MAPK
Intracellular p38 MAPK activity was detected using p38 MAP kinase assay kit (Cell Signalling Technology). Equal amounts of cytoplasmic protein from each group were used for the immunoprecipitation procedures. Fifteen microliters of anti-phospho-p38 monoclonal antibody immobilized by cross-linkage to agarose hydrazide beads was added to each lysate and incubated overnight at 4°C. The immune complexes were collected by centrifugation and then washed extensively on the following day. Immunoprecipitates were resuspended in 25 μL of kinase buffer supplemented with 200 μmol/L ATP and 2 μg ATF-2 fusion protein (Cell Signaling Technology) and incubated for 1 h at 30°C. After incubation, the reaction was terminated using Laemmli buffer (62.5 mmol/L Tris–HCl, pH 6.8, 10% glycerol, 2% SDS, 5% β-mercaptoethanol). The samples were boiled for 5 min and loaded onto a 10% SDS-polyacrylamide gel and probed with anti-phospho-ATF-2 rabbit polyclonal antibody (Cell Signaling Technology, 1:1000).
Electrophoretic mobility shift assay
DNA binding activity of NF-κB was determined by electrophoretic mobility shift assay (EMSA) with Gel shift assay systems (Promega, Beijing, China), according to manufacturer’s instructions. Double-stranded oligonucleotides containing the consensus sequence for NF-κB/DNA binding site (5'-TAGTTGAGGGGACTTTCCCAGG-3') were radiolabeled with [γ-32P]-ATP (Amersham Biosciences, Little Chalfont, Bucks, UK). After purification over a Sephadex G-25 column (Amersham Biosciences), 32P-labeled oligonucleotides (20 000 r/min) were incubated with nuclear extracts at room temperature for 20 min in binding buffer containing 12 mmol/L HEPES (pH 7.9), 4 mmol/L Tris HCl (pH 7.9), 60 mmol/L KCl, 1 mmol/L EDTA, 1 mmol/L DTT, 1 mmol/L PMSF, 12% glycerol, 5 μg of BSA, and 2 μg of poly (dI/dC) poly (dI/dC) (Amersham Biosciencs). DNA-protein complexes were separated in 6% nondenaturing polyacrylamide gel. Signals were visualized by exposing the dried gel to X-ray film.
Northern blot analysis
Total RNA was isolated from whole murine liver tissue by the guanidinium isothiocyanate/cesium chloride procedure. Complementary DNA probes were obtained by reverse transcriptase-polymerase chain reaction (RT-PCR) with specific primers, labeled with [α-32P] dCTP by random priming (Megaprime DNA labeling system, Amersham Biosciences). The sequences of the oligonucleotide primers used for PCR were designed using Primer 5.0 software as follows: mouse IFN-γ sense: 5'-AGCGGC TGACTGAACTCAGATTGT AG-3', antisense: 5'-GTCACAGTTTTCAGCTGTATAGGG-3', mouse IL-12 p40 sense: 5'-CAGAAGCTAACCATCTCCTGG TTTG-3, antisense: 5'-TCCGGAGTAATTTGGTGCTTCACAC-3', mouse IL-1β sense prime: 5'-GCAACTGTTCCTGAACTCA-3', antisense prime: 5'-CTCGGAGCCTGTAGTGCAG-3', mouse TNFα sense prime: 5'-GGCAGGTCTACTTTG GAG TCA TTG C-3', antisense prime: 5'-ACATTCGAGGCTC CAGTGAATTCGG-3', mouse β-actin sense prime: 5'-TGGAATCCTGTGGCATCCATGAA, antisense prime: 5'-TAAAACGCAGCTCAGTAACAGTCCG-3'. Thirty cycles of amplification were performed: denaturation at 94°C for 60 s, annealing at 57°C for 60 s, and extension at 72°C for 60 s. The identity of PCR products was confirmed by direct sequencing. For Northern blots, 10 μg of total RNA was fractionated on 1.2% formaldehyde-agarose gels and blotted onto Hybond-N+ nylon membranes (Amersham Biosciences), which were hybridized separately with individual probes overnight at 45°C in a solution containing 50% formamide, 5 × SSC, 2.5 × Denhardt's solution, 25 mmol/L sodium phosphate buffer (pH 6.5), 0.1% SDS, and 250 μg/mL salmon sperm DNA. All Northern blots were subjected to stringent washing conditions prior to autoradiography with intensifying screen at -80°C for 2-7 d. Cytokine mRNA expressions were normalized to internal standards for 2 different constitutively expressed mRNAs (β-actin).
Statistical analysis
All data were expressed as mean ± SE. Differences between groups were assessed by one way ANOVA analysis. P < 0.05 was considered statistically significant. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) statistical software for Windows, version 101.4 (SPSS Inc., Chicago, IL, USA).
RESULTS
Activation of p38 MAPK and NF-κB in EAH
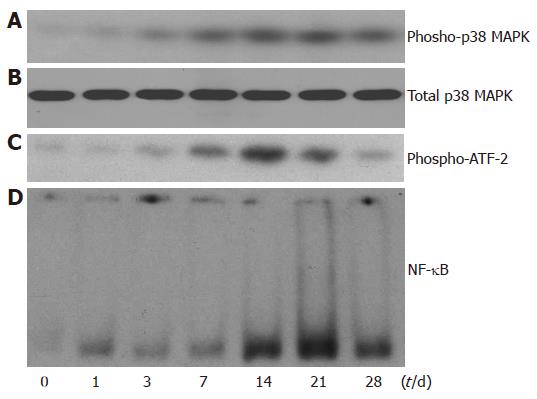
In the current study, we observed the activation of p38 MAPK on d 1, 3, 7, 14, 21, 28 after the first immunization. The expression of phospho-p38 MAPK was progressively increased after injection of syngeneic S-100 antigen and reached its peak on d 14 to 21 after the first immunization (Figure 1A). The expression of total p38 MAPK, however, remained constant (Figure 1B), indicating that the activity of p38 MAPK was increased. To confirm our finding, we also determined the activity of p38 MAPK by immunoprecipitate kinase activity assay. As shown in Figure 1C, the p38 MAPK activity was increased and reached its peak on d 14 to 21 after administration of syngeneic S-100 antigen. These results showed that p38 MAPK activity was increased in mouse liver after IAH was induced by auto-antigens.
Figure 1 Increased activity of p38MAPK and NF-κB in EAH liver.
A: Expression of phosphor-p38 MAPK protein by Western blot in whole liver extract; B:Expression of total p38 MAPK protein by Western blot in whole liver extract; C: Immunoprecipitate kinase activity assays of p38 MAPK; D: Electrophoretic mobility shift assay of NF-κB DNA binding activity using nuclear protein extracts from EAH liver.
NF-κB DNA binding activity increased significantly after treatment with syngeneic S-100 antigen and reached its peak on d 21 after the first immunization (Figure 1D), consistent with that of p38 MAPK.
Suppression of p38 MAPK and NF-κB activities in EAH by SB203580
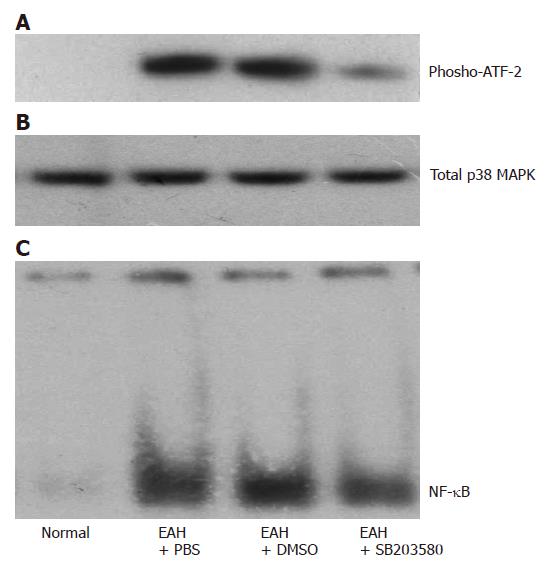
Because the activity of p38 MAPK and NF-κB was increased in mice with EAH, we tried to find whether SB203580, a specific p38 MAPK inhibitor, would reduce their activity and whether SB203580 would improve hepatic inflammation in this model. SB203580 significantly reduced the activity of p38 MAPK as reflected by reduced phosphor-ATF-2 in immunoprecipitate kinase activity assay (Figure 2A). Similarly, the DNA binding activity of NF-κB also decreased significantly in SB203580-treated group.
Figure 2 Effects of SB203580, a specific p38 MAPK inhibitor, on the activation of p38 MAPK and NF-κB 14 d after the first immunization.
A: Representative immunoprecipitate kinase activity assays of p38 MAPK using protein extracts from normal control, EAH mice treated with PBS, DMSO, and SB 203580, respectively; B: Western bolt of total p38 MAPK; C: Representative electrophoretic mobility shift assays of NF-κB DNA binding activity using nuclear protein extracts from liver of the 4 study groups as in A.
Reduction in expression of pro-inflammatory cytokines after SB 203580 treatment
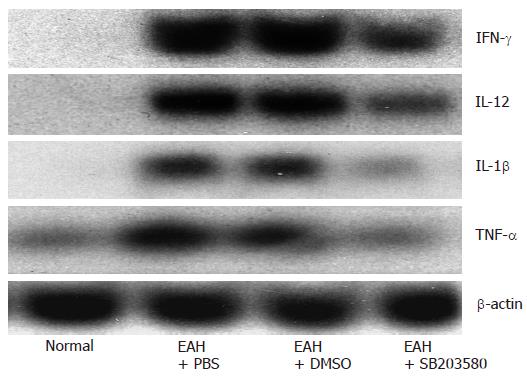
Since Th1 cytokines play a main role in adult[20] and children patients[21] with typeIAIH, we investigated the expression of Th1 cytokine mRNA in the setting of SB203580 treatment which significantly reduced IFN-γ, IL-12, TNF-α and IL-1β expression in livers of EAH mice. Our results showed that Th1 cytokines (IFN-γ, IL-12, IL-1β) and TNF-α mRNA were up-regulated in EAH (Figure 3).
Figure 3 Effects of SB203580, a specific p38 MAPK inhibitor, on the expression of proinflammatory cytokines.
Northern blot of IFN-γ, IL-12, IL-1β and TNF-α from total liver RNA isolated from normal control, EAH mice treated with PBS, DMSO, and SB203580, respectively. The same blot was striped and reprobed with β-actin as an internal loading control.
Improvement in hepatic inflammation and injury in EAH after SB203580 treatment
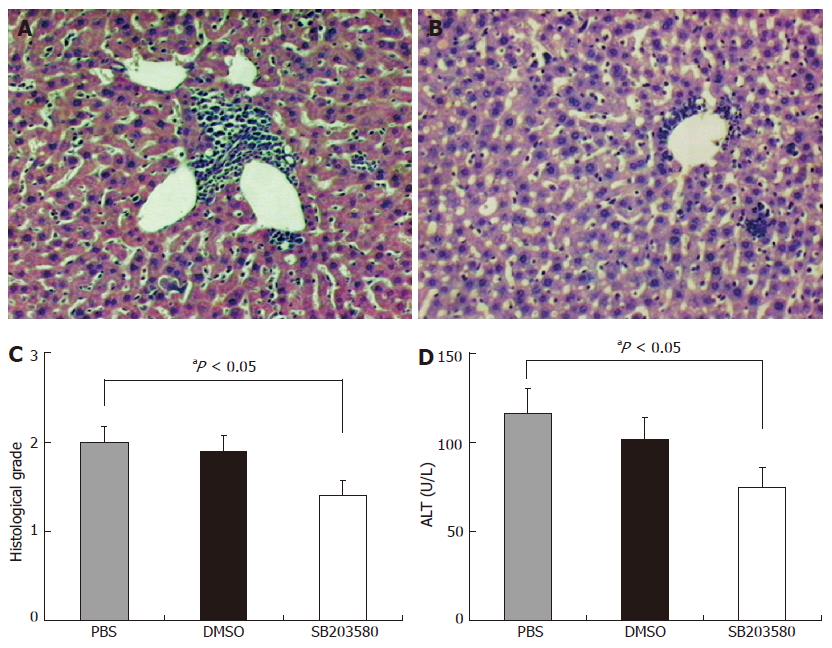
We observed the effect of SB203580 on hepatic inflammation in this animal model. After given syngeneic S-100 antigen, the mice developed perivascular infiltrates as well as intralobular inflammatory and necrotic lesions (Figure 4A). SB203580 significantly attenuated perivascular infiltrates as well as intralobular inflammation or necrosis (Figure 4B). The severity of inflammation as reflected by histological inflammation score was markedly decreased from 2.0 ± 0.18 in the control group to 1.4 ± 0.18 in the SB203580-treated group (Figure 4C). SB203580 decreased the serum ALT level from 116 ± 14 U/L in PBS control group and 103 ± 11 U/L in vehicle control group to 75 ± 10 U/L in SB203580-treated group (Figure 4D). DMSO-treated animals did show the same histological picture of typical EAH as PBS- treated animals.
Figure 4 Effects of SB203580, a specific p38 MAPK inhibitor, on hepatic inflammation in EAH.
A: Representative histology of control animals after standard induction of EAH showing portal inflammation (grade 2); B: Representative histology of animals treated with SB203580 after standard induction of EAH showing significantly reduced portal inflammation (grade 1); C: Average histological inflammation grade score for PBS, DMSO and SB203580 treated animals after standard induction of EAH (aP < 0.05 between PBS and SB203580 treated animals); D: Serum alanine transaminase (ALT) levels in PBS, DMSO and SB203580 treated animals after standard induction of EAH (aP < 0.05 between PBS and SB203580 treated animals).
DISCUSSION
Our previous study and other studies showed that intraperitoneal injection of hepatic S100 antigen causes inflammatory cell infiltration and hepatocelluar injury, which was accompanied with a mild increase in alanine aminotransferase (ALT) level, and peaked at 4 wk after the first immunization[3,18,19].
In this study, we investigated the role of p38 MAPK in an animal model of autoimmune liver injury. The activation of p38 MAPK signaling pathway was up-regulated in experimental autoimmune hepatitis, and the inhibition of p38 MAPK reduced hepatic inflammation and injury. The protection against hepatic injury induced by p38 MAPK was associated with a diminished expression of NF-κB and Th1 cytokines (IFN-γ, IL-12, IL-β and TNF-α) known to promote hepatic injury in AIH, showing that activation of p38 MAPK plays an important role in promoting cytokine/chemokine production, which in turn results in autoimmune hepatic injury in EAH.
The p38 MAPK signaling transduction pathway plays an essential role in regulating many cellular processes, including inflammation, cell differentiation, cell growth and death[22]. Activation of the p38 MAPK pathway plays an essential role in the production of proinflammatory cytokines[23]. By early discovery of the p38 MAPK signaling pathway, specific inhibitors of p38 MAPK could be identified. Pyridinyl imidazole compounds were found to inhibit the production of TNF-α and IL-1 in lipopolysaccharide (LPS)-stimulated cells. These compounds can bind to a protein that has been proved to be p38 MAPK[24]. The most widely used agents are SB203580 and SB202190. Because inhibiting p38 MAPK suppresses production of key mediators, it is an obvious target for the treatment of chronic inflammatory disease[25]. In addition, MAP kinases play an important role in immune responses from the innate to the adaptive immune system, from the initiation of immune responses to activation-induced cell death[26]. Recently, Hollenbach and collegues[11] found that SB203580 improves the clinical score, ameliorates histological alterations, and reduces mRNA levels of proinflammatory cytokines in dextran sodium sulphate (DSS)-induced experimental colitis model of mice. These results suggest that p38 MAPK is a therapeutic target for autoimmune diseases. In our study, the activation of p38 MAPK was found to increase in a time-dependent manner in mice with experimental autoimmune hepatitis, and reached its peak on d 14 after the first syngeneic S-100 administration, preceding that of histological lesions (about at 4 wk). Furthermore, inhibition of p38 MAPK activity by SB203580 administration could decrease the serum ALT level and improve hepatic lesion significantly. As expected, SB203580 ccould also inhibit the expression of Th1 proinflammatory cytokines (IFN-γ, IL-12, IL-1β and TNF-α), which are the key cytokines in inflammatory response. These results suggest that activation of p38 MAPK is one of the initial pathogenic factors for autoimmune liver injury and its inhibition provides a novel therapeutic strategy.
It was reported that Th1cytokines play a main role in the pathogenesis of typeIAIH[20,21,27]. Hussain et al[28] showed that TNF-α and IFN-γ producing cells are detectable in inflammatory cell infiltrates, revealing a significant correlation between the frequency of TNF-α and IFN-γ producing cells, the intensity of inflammatory cell infiltrates and transaminase level. Recently, Chernavsky et al[21] showed that expression of IFN-γ and IL-12 is up regulated in liver, but is not detectable in control liver. In addition, Czaja et al[29] and Cookson et al[30] demonstrated that patients with type 1 AIH have a higher frequency than those with a normal TNF gene polymorphism associated with increased transcription of TNF-α. Patients with such a polymorphism have a lower frequency of remission during corticosteroid therapy and a higher occurrence of treatment failure and cirrhosis. Our results indicate that inhibition of the expression of Th1 cytokines could result in remission of EAH.
NF-κB is a pivotal transcription factor for the regulation of many genes, particularly those for inflammatory and immune responses, including IL-1β, IL-12 and TNF-α[31,32]. Because a large variety of stimulations activate NF-κB and the transcription factor regulates the expression of inflammatory cytokines, chemokines, immunoreceptors, and cell adhesion molecules, NF-κB is often termed a central mediator of human immune response[33]. It has been reported that inhibition of NF-κB activation in vivo, using a selective proteasome inhibitor, attenuates chronic inflammation in experimental models of Crohn’s disease and rheumatoid arthritis[34,35]. Previous studies have clearly demonstrated that NF-κB might be an effector of p38 MAPK[36]. We found that NF-κB activation was also induced in mice with EAH, and reached its peak a little later than p38 MAPK. Furthermore, inhibition of p38 MAPK activation could reduce the activation of NF-κB, suggesting that p38 MAPK upregulation promotes release of inflammatory cytokines via a NF-κB-dependent mechanism in autoimmune liver injury.
In summary, p38 MAPK plays an important role in liver injury induced by autoimmunity, and inhibition of p38 MAPK attenuates EAH, thus providing a biochemical basis for the potential of using specific inhibitors of p38 MAPK for treating autoimmune hepatitis.