Published online Aug 21, 2007. doi: 10.3748/wjg.v13.i31.4214
Revised: March 15, 2007
Accepted: March 21, 2007
Published online: August 21, 2007
AIM: To construct pcDNA3.1-Egr.1p-p16 recombinant plasmid and investigate the expression of p16 in pancreatic cancer JF305 cells induced by radiation and the feasibility of gene radiotherapy for pancreatic carcinoma.
METHODS: Human p16 cDNA was ligated to the downstream of Egr-1 promotor to construct pcDNA3.1-Egr.1p-p16 plasmid by restriction enzyme digested. The recombined plasmids were transfected into pancreatic cancer JF305 cells with lipofectamine. p16 mRNA level was detected by RT-PCR. The expression of p16 after different doses of X-ray radiation was detected by Western blot technique. Cell survival was assessed by clonogenic assays and cell viability was analysed by trypen blue exclusion. Flow cytometry was performed to study the apoptosis of JF305 cells.
RESULTS: Restriction enzyme digestion showed the correctly constructed pcDNA3.1-Egr.1p-p16. The p16 expression in cells transfected with pcDNA3.1-Egr.1p-p16 induced by different doses of radiation was higher than that in the control group (P < 0.05). Eight hours after 2 Gy X-ray radiation, the expression reached its peak (87.00 ng/L), and was significantly higher than that in the control group (P < 0.0.5). Clonogenic analysis and trypan blue extraction test showed that the pcDNA3.1-Egr.1p-p16 transfer enhanced radiation-induced cell killing in p16-null JF305 cell lines. The induction of apoptosis was lower in combined transfection and irradiation group than that in irradiation alone.
CONCLUSION: X-ray can induce the recombinant plasmid pcDNA3.1-Egr.1p-p16 expression in JF305 cells. The detection of dose and time provides an experimental basis for in vivo study in future.
-
Citation: Ma HB, Wang XJ, Di ZL, Xia H, Li Z, Liu J, Ma J, Kang HF, Wu CM, Bai MH. Construction of targeted plasmid vector pcDNA3.1-Egr.1p-p16 and its expression in pancreatic cancer JF305 cells induced by radiation
in vitro . World J Gastroenterol 2007; 13(31): 4214-4218 - URL: https://www.wjgnet.com/1007-9327/full/v13/i31/4214.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i31.4214
While traditional surgery, chemotherapy and radiation therapy conveyed significant cure rates for many types of cancer, the results are less encouraging for other types due to the inability to optimally localize the activity of therapeutic agents to tumor cells and limit damage to normal tissue, thus allowing curative doses to be delivered. This can be improved as recent advances in gene therapy, in which transgene delivered by viral or non-viral vectors is introduced into tumor cells, can potentially provide new means of targeting toxicity such as selective transgene activation[1-3]. The delivery of therapeutic genes into cancer cells or normal tissues of cancer patients is an important strategy in cancer therapy[4]. Previous studies have shown that the six 5’ CarG [CC(A+T rich) GG] elements mediate transcriptional induction of the Egr-1 gene promoter following ionizing radiation. Transcriptional activation of the Egr-1 gene by ionizing radiation appears to be a direct consequence of the production of reactive oxygen intermediates. It is an effective approach that uses the specificity of the Egr-1 promoter to construct the radiation-inducible gene expression system with destination gene[5-7]. In 1994, Weichselbaum linked DNA sequences from the promotor region of Egr-1 to a complementary DNA sequence which encodes human tumor necrosis factor alpha (TNF-α). By transfecting Egr-TNF into a human HL525 cell line, combined gene and radiation therapy can enhance tumor cure rate without increasing toxicity in normal tissue and is a new paradigm for cancer treatment[8]. The p16 tumor suppressor gene is localized to chromosome 9p21 and its protein product inhibits cyclin D and CDK4/6 in G1. Restraint of cell division cycle in cell proliferation defines p16 as a negative regulation factor for cell division[9-11]. To study the feasibility of radiation inducible gene therapy, we constructed the CarG elements of the Egr-1 promoter- ligated upstream of cDNA for p16 (pcDNA3.1-Egr.1p-p16) and investigated the expression of p16 in pancreatic cancer JF305 cells induced by radiation.
Human pancreatic cancer JF305 cells were obtained from China Medical University. Cells were grown in RPMI 1640 (GIBCO Company, Grand Island, NY, USA).The medium was supplemented with 10% fetal calf serm, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were incubated at 37°C in a humidified atmosphere of 5% CO2 and sub-cultured when confluence was reached. The medium was changed every 5 d.
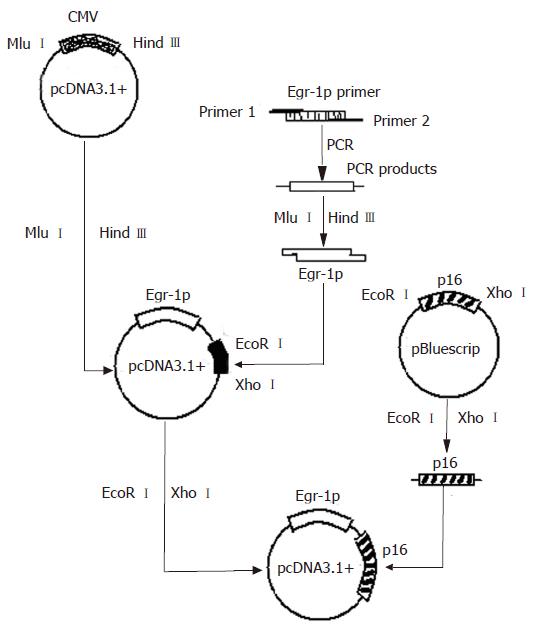
pMD18-T and Egr-1p were kindly provided by Wu Cong-Mei (Shantou University Medical College, MD). Egr-1 promoter was derived by PCR amplification from the plasmid pMD18-T.Egr-1p using primers (5'-TGAACGCGTGACCCGGAAACGCCATATAAG-3' and 5'-ATTAAGCTTCCAAGTTCTGCGCGCTGGGATC-3'). Amplification was performed for 35 cycles at 95°C for 45 s, at 60°C for 45 s and at 72°C for 1 min. p16 was kindly provided by Professor Zhang Bo (Beijing University Medical College). pcDNA3.1+ vector was purchased from Invitrogen (Carlsbad, California, USA). Expression vector for p16 was constructed in our laboratory (Figure 1).
A total of 5 × 105 JF305 cells were seeded into each well of a six-well plate. On the third day (when the cells reached 80%-90% confluence), the culture medium was aspirated, and the cell monolayer was washed with pre-warmed sterile PBS. Cells were transfected with the appropriate construct using Lipofectamine 2000 according to the manufacturer’s instructions. Four micrograms of plasmid DNA (in 10 μL ddH2O) was mixed with 250 μL of serum-free medium(SFM) and incubated for 20 min at room temperature with a mixture of 10 μL Lipofectamine 2000 and 250 μL SFM and the resultant complex mixture was added to a monolayer of pre-confluent cells seeded in a six-well plate. The medium was replaced with fresh and complete one 6 h after transfection. The cells were exposed to irradiation 36 h after transfection.
Cell monolayer was irradiated at 37°C using a Varian Clinac 600C linear accelerator at a dose rate of 2.5 Gy/min for 0, 2, 4, 8, 10 and 20 Gy groups, respectively.
Cells were treated with lysis buffer containing 1% NP40, 137 mmol/L NaCL, 20 mmol/L Tris base(pH 7.4), 1 mmol/L DTT, 10% glycerol, 10 mg/mL Aprotinin, 2 mmol/L sodium vanadate and 100 μmol/L PMSF. Protein concentrations were determined using the PIERCE BCA protein assay kit. Protein was separated by 10% SDS-PAGE under denaturing conditions and transferred to nitrocellulose membranes. Membranes were incubated with mouse p16 monoclonal antibody (1:1000; Santa Cruz Biotechnology), followed by incubation in goat anti-mouse secondary antibody conjugated with horseradish peroxidase (1:1000; Santa Cruz Biotechnology). Immunoreactive proteins were detected with enhanced chemiluminescence detection system (Amersham Biosciences)
Total RNA was prepared using Trizol reagent as described by the manufacturer (Life Technologies, Inc). One μg of total RNA was reverse transcribed by Moloney murine leukemia virus reverse transcriptase (Promega) under standard conditions. Duplicate samples of 1 μL each cDNA were amplified by PCR. The amplification reaction mixture (50 μL) contained cDNAs (p16: forward primer 5'-CCTCGTGCTGATGCTACTGA-3' and reverse primer 5'-CATGGTTACTGCCTCTGGTG-3'; GAPDH: forward primer 5'-TCAACGGCACAGTCAAGG-3' and reverse primer 5'-TGAGCCCTTCCACGATG-3'). GAPDH was used as an internal control to normalize variable amounts of cDNA in each sample. A product with 241 bp was generated, and PCR products were analyzed on 15 g/L agarose gel. The assay for each sample was repeated at least twice.
Cell survival was assessed by clonogenic assay in monolayer culture. In brief, the cells were trypsinised, counted, placed in the glass tube and irradiated. The known number of cells was then re-plated in 100-mm culture dishes and returned to the incubator to allow macroscopic colony development. The surviving fractions following a given treatment were calculated based on the survival of non-irradiated non-transfected cells.
The viability of cells with or without irradiation (4 Gy) was analyzed using trypan blue exclusion. Each experiment was repeated at least twice.
Apoptosis was quantified by flow cytometry using the APOBRDUTM kit (PharMinge, San Diego, CA, USA). In brief, 2 × 106 cells were fixed with 1% paraformaldhyde in phosphate-buffered saline (PBS) for 15 min at room temperature, washed twice with PBS, and stored in 70% ethanol at -20°C. Flow cytometry analysis was performed with a EPICS flow cytometer (Coulter Corp, Hialeah, FL, USA). Histograms of cell number logarithmic fluorescence intensity were recorded for 10 000 cells per sample.
All experiments were performed at least three times. Student’s t and correlation tests were used to determine the comparability of groups. P < 0.05 was considered statistically significant.
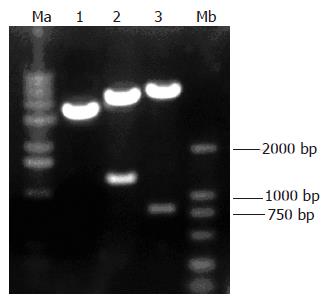
The reconstructed plasmid was identified by digestion with restriction enzymes MluI/HindIII and EcoRI/XhoIrespectively and the digestive products were visualized on 10 g/L agarose gel (Figure 2), demonstrating that recombinant plasmids were digested to 1300 bp and 800 bp DNA fragments, which carried the target gene Egr-1p and p16.
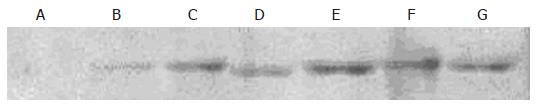
JF305 cells transfected with pcDNA3.1-Egr.1p-p16 were irradiated by different doses of X-ray. The cells in the control group were transfected with pcDNA3.1+. Eight hours after irradiation, the protein was extracted and the expression of p16 was detected by Western blot. The results showed no p16 expression in the control group and higher p16 expression in 2, 4, 8, 10 and 20 Gy groups than in 0 Gy group (Figure 3).
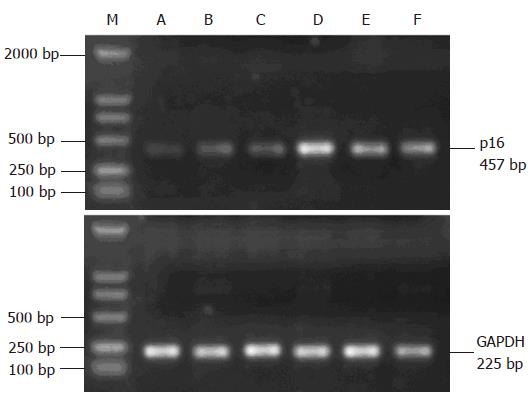
The expression of p16 gene after X-irradiation was detected by RT-PCR. Control RNA from un-irradiated cells demonstrated a low but a detectable level. In contrast, p16 expression increased in a dose-dependent manner in irradiated cells (Figure 4).
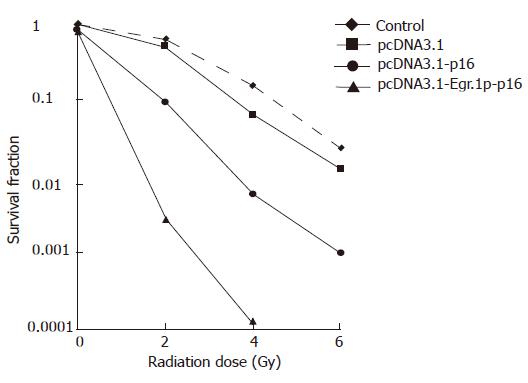
We first tested whether pcDNA3.1-Egr-1p and p16 transfection increased the radio-sensitivity of JF305 pancreatic carcinoma cell lines in vitro. Clonogenic assays were performed on JF305 cells. The survival of non-irradiated JF305 cells increased 86% and 78% respectively after pcDNA3.1-p16, pcDNA3.1-Egr-1p-p16 transfection, while reduced from 63% to 9.22% and 0.28% at 2 Gy, from 15% to 0.59% and 0.012% at 4 Gy (Figure 5). PcDNA3.1 transfection alone did not produce marked sensitization in JF305 cells in the range of 2-6 Gy. JF305 cells transfected with pcDNA3.1-Egr.1p-p16 were much more radiosensitive than non-transfected or pcDNA3.1-p16 transfected cells.
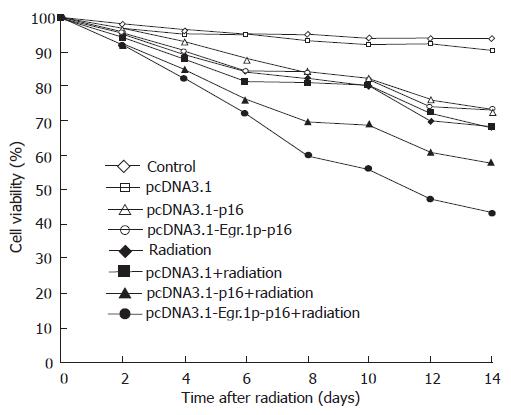
Next, we examined the viability of the JF305 cells using the trypan blue exclusion test (Figure 6). Six days after radiation, no difference was found in viability of pcDNA3.1-Egr-1p-p16–transfeced cells and pcDNA3.1-p16 transfected or non-transfected cells. Six days after irradiation, the viability of irradiated pcDNA3.1-Egr-1p-p16 transfeced cells gradually decreased, compared with pcDNA3.1-p16 transfected, non-transfected and non-irradiated cells. The viability of irradiated pcDNA3.1-Egr-1p-p16 transfeced JF305 cells decreased more significantly than that of pcDNA3.1-p16 transfected cells (χ2 = 3.92, P < 0.05 on d 12; χ2 = 4.46, P < 0.05 on d 14).
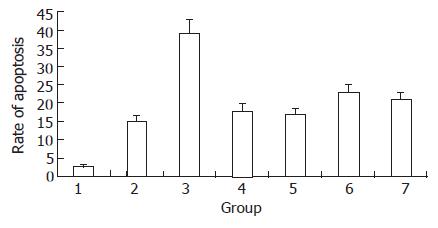
Only a minor fraction of cells in the control group underwent apoptosis (3%). However, when cells were irradiated with a dose of 2 Gy, the proportion of apoptotic cells reached 39%, which was higher than that in pcDNA3.1+ group (15%, P < 0.01). In JF305 cells transfected with pcDNA3.1-p16 or pcDNA3.1-Egr-1p-p16, the apoptosis rate was 18% and 17%. However, when the cells were exposed to 2 Gy irradiation, the apoptosis rate was 23% and 21%, being lower than that in the irradiation group (39%, P < 0.05) (Figure 7).
Pancreatic cancer remains a challenge to the oncologist. The death to incidence ratio of pancreatic cancer is approximately 0.98-0.99:1[12,13]. More than 27 000 people die of pancreatic cancer each year and it is the fourth leading cause of cancer death in the United States[14]. Some reports have shown that the mortality of pancreatic cancer in China has increased constantly over the last decades[15-18]. With the rapid development of molecular biology, gene radiotherapy has been considered as an effective cancer treatment. Weichselbaum et al[8] are the forerunners in tumor gene radiotherapy (Egr-TNF). Since they put forward that ionizing radiation could be used to activate the transcription of exogenous genes encoding cytotoxic proteins, and established the techniques that might be used to target gene therapy during the treatment of human neoplasms in 1992, a variety of downstream genes have been introduced to Egr-1 promoter for the treatment of different tumors.
The p16 gene maps on 9p21 contain three exons and encode a nuclear phosphoprotein with a molecular weight of 16 kDa. The p16 protein negatively regulates the cell cycle by inhibiting cyclin-dependent kinases 4 and 6 and interacting with cyclin D1. In the absence of p16, CDKs bind to cyclin D1 and retinoblastoma protein(pRb) is phosphorylated, leading to its deregulation at the G1/S checkpoint, and cell proliferation is switched on. In a variety of human malignant tumors and cell lines, the p16INK4A gene is inactivated by various genetic mechanisms, including point mutation, homozygous deletion, and hypermethylation of CpG islands in the p16INK4A promoter[19]. p16 seems to act in a regulatory feedback circuit with CDK4, D-type cyclin and retinoblastoma protein. Over-expression of p16 gene could block cell cycle progression through the G1-to-S phase boundary in a pRB-dependent manner. These findings suggest that the CDK4- inhibitory activity of p16 is involved in regulating cell cycle progression through the G1/S boundary[20].
Based on the anticancer action of p16, we constructed pcDNA3.1-Egr.1p-p16 plasmid and transfected JF305 cells to study the expression properties of the plasmid induced by ionizing irradiation. The results revealed that the p16 expression in cells transfected with pcDNA3.1-Egr.1p-p16 induced by irradiation with 2, 4, 8, 10 and 20 Gy X-ray was higher than that in the sham-irradiation group. JF305 cells transfected with pcDNA3.1-Egr.1p-p16 were much more radiosensitive than nontransfected or pcDNA3.1-p16 transfected cells. The viability of irradiated pcDNA3.1-Egr-1p-p16 transfeced JF305 cells decreased more significantly than that of pcDNA3.1-p16 transfected cells (χ2 = 3.92, P < 0.05 on d 12; and χ2 = 4.46, P < 0.05 on d 14). Combination of pcDNA3.1-Egr-1p-p16 and radiation could induce apoptosis of JF305 cells, but the apoptosis rate was not very high.
The mortality of pancreatic carcinoma is still high in the world, and its treatment is still difficult[21]. Our study has laid some theoretical basis for further study on pancreatic carcinoma gene-radiotherapy.
S- Editor Liu Y L- Editor Wang XL E- Editor Li JL
| 1. | Hsu H, Rainov NG, Quinones A, Eling DJ, Sakamoto KM, Spear MA. Combined radiation and cytochrome CYP4B1/4-ipomeanol gene therapy using the EGR1 promoter. Anticancer Res. 2003;23:2723-2728. [PubMed] |
| 2. | Scott SD, Marples B, Hendry JH, Lashford LS, Embleton MJ, Hunter RD, Howell A, Margison GP. A radiation-controlled molecular switch for use in gene therapy of cancer. Gene Ther. 2000;7:1121-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Thiel G, Cibelli G. Regulation of life and death by the zinc finger transcription factor Egr-1. J Cell Physiol. 2002;193:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 483] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 4. | Weichselbaum RR, Kufe DW, Advani SJ, Roizman B. Molecular targeting of gene therapy and radiotherapy. Acta Oncol. 2001;40:735-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Tsai-Morris CH, Cao XM, Sukhatme VP. 5' flanking sequence and genomic structure of Egr-1, a murine mitogen inducible zinc finger encoding gene. Nucleic Acids Res. 1988;16:8835-8846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 128] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Datta R, Taneja N, Sukhatme VP, Qureshi SA, Weichselbaum R, Kufe DW. Reactive oxygen intermediates target CC(A/T)6GG sequences to mediate activation of the early growth response 1 transcription factor gene by ionizing radiation. Proc Natl Acad Sci USA. 1993;90:2419-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 124] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Manome Y, Kunieda T, Wen PY, Koga T, Kufe DW, Ohno T. Transgene expression in malignant glioma using a replication-defective adenoviral vector containing the Egr-1 promoter: activation by ionizing radiation or uptake of radioactive iododeoxyuridine. Hum Gene Ther. 1998;9:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Weichselbaum RR, Hallahan DE, Beckett MA, Mauceri HJ, Lee H, Sukhatme VP, Kufe DW. Gene therapy targeted by radiation preferentially radiosensitizes tumor cells. Cancer Res. 1994;54:4266-4269. [PubMed] |
| 9. | Li J, Qin D, Knobloch TJ, Tsai MD, Weghorst CM, Melvin WS, Muscarella P. Expression and characterization of Syrian golden hamster p16, a homologue of human tumor suppressor p16 INK4A. Biochem Biophys Res Commun. 2003;304:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Cowgill SM, Muscarella P. The genetics of pancreatic cancer. Am J Surg. 2003;186:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Gerdes B, Ramaswamy A, Ziegler A, Lang SA, Kersting M, Baumann R, Wild A, Moll R, Rothmund M, Bartsch DK. p16INK4a is a prognostic marker in resected ductal pancreatic cancer: an analysis of p16INK4a, p53, MDM2, an Rb. Ann Surg. 2002;235:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Devesa SS, Blot WJ, Stone BJ, Miller BA, Tarone RE, Fraumeni JF. Recent cancer trends in the United States. J Natl Cancer Inst. 1995;87:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 266] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Pisani P, Parkin DM, Bray F, Ferlay J. Erratum: Estimates of the worldwide mortality from 25 cancers in 1990. Int. J. Cancer, 83, 18-29 (1999). Int J Cancer. 1999;83:870-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2959] [Cited by in RCA: 2761] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 15. | Cao L, Liu D. Diagnosis and treatment of pancreatoblastoma in China. Pancreas. 2007;34:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Wang L, Yang GH, Li H, Lu XH. The changing pancreatic cancer mortality in China (1991-2000). Zhonghua NeiKe ZaZhi. 2005;44:509-513. [PubMed] |
| 17. | Guo X, Cui Z. Current diagnosis and treatment of pancreatic cancer in China. Pancreas. 2005;31:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Zhang QH, Ni QX. Clinical analysis of 2340 cases of pancreatic cancer. Zhonghua YiXue ZaZhi. 2004;84:214-218. [PubMed] |
| 19. | Kim YT, Zhao M. Aberrant cell cycle regulation in cervical carcinoma. Yonsei Med J. 2005;46:597-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Wu CM, Huang TH, Xie QD, Wu DS, Xu XH. Construction of pETNF-P16 plasmid and its expression properties in EC9706 cell line induced by X-ray irradiation. World J Gastroenterol. 2004;10:2927-2930. [PubMed] |
| 21. | Bergenfeldt M, Albertsson M. Current state of adjuvant therapy in resected pancreatic adenocarcinoma. Acta Oncol. 2006;45:124-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |