Published online Aug 14, 2007. doi: 10.3748/wjg.v13.i30.4149
Revised: May 3, 2007
Accepted: May 12, 2007
Published online: August 14, 2007
Interferon (IFN) therapy is the only treatment strategy for hepatitis C virus (HCV) infection after liver transplantation (LT), but prophylactic and treatable IFN therapy after LT has been shown to be insufficient due to the adverse effects of IFN and rivabirin. In this paper, we describe the disappearance of HCV after LT without IFN therapy in the presence of residual viremia on the day of LT. We herein report our findings since this is considered an important case for the anti-HCV strategy of post LT. A 60-year old woman with LC and HCC was referred to Nagasaki University Hospital in August 2004. After she underwent LT on February 18, 2005, we injected peg-IFN-α-2a the 11th time at 18 wk and HCV-RNA was still positive in the serum at LT. The serum HCV-RNA was negative one month after operation and subsequently dissolved 15 mo after operation without IFN therapy. As a result, we speculate that if HCV-RNA is positive while HCV core antigen is negative before LT, then it may lead to clearance of HCV after LT. Therefore long acting peg-IFN-α-2a is thus considered a potentially effective agent for the treatment of HCV-related cirrhosis before LT.
- Citation: Ichikawa T, Nakao K, Hamasaki K, Honda T, Shibata H, Akahoshi M, Eguchi S, Takatsuki M, Kanematsu T, Eguchi K. Clearance of hepatitis C virus after living-donor liver transplantation in spite of residual viremia on end date of interferon therapy before transplantation. World J Gastroenterol 2007; 13(30): 4149-4151
- URL: https://www.wjgnet.com/1007-9327/full/v13/i30/4149.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i30.4149
Living donor liver transplantation (LDLT) has become a common treatment strategy for hepatocellular carcinoma (HCC) and end stage liver cirrhosis (LC) in Japan[1]. However, hepatitis C virus (HCV) infection, the most common cause of LDLT, is found in nearly all re-infected graft livers, thus leading to a rapid progression to LC and re-liver transplantation[2]. Interferon (IFN) treatment for HCV infection after LT is the only treatment strategy at present, but its effects are still incomplete[3]. Because the titer of HCV is the most decay in early transplant phase of liver transplantation[4], anti-HCV therapy could thus be considered at this time[5], but prophylactic and treatable IFN therapy after LT has so far been ineffective due to the adverse effects of IFN and rivabirin[6].
Recently, pegylated interferon (peg-IFN), utilizing polyethyleneglycol moiety attached to IFN via an amide bond, has been used in the treatment of chronic HCV infection. Peg-IFN-α-2a characterized by a prolonged absorption half-life (50 h), a restricted volume of distribution (8-12 L), and a decreased clearance (94 mL/h)[7], has been found to be safe and tolerable after LT[8]. We thus consider peg-IFN-α-2a a potentially useful treatment of LC due to HCV infection in patients awaiting liver transplantation.
In this case, we made an attempt to achieve HCV clearance from a graft liver after LDLT. For this purpose, peg-IFN-α-2a mono-therapy was performed for 13 wk until LDLT. We observed the disappearance of HCV after LDLT without IFN therapy in the presence of residual viremia on the day of LDLT. The titer of HCV disappeared in early post-LDLT due to the administration of peg-IFN-α-2a. We herein report our findings.
A 60-year old woman with LC and HCC was referred to Nagasaki University Hospital in August 2004. She was diagnosed having diabetes and HCV-related LC in 1995 and 1999, respectively, and had no history of blood transfusion, alcohol abuse and intra-venous drug use. After the diagnosis of LC, she was treated with IFN-α, but this medication was stopped due to depression. In August 2003, a tumor was detected measuring 3.5 cm in diameter in the caudate lobe of the liver, she underwent trans-arterial chemoembolization (TACE) therapy twice in September 2003 and February 2004. The HCC decreased and no new HCC was detected. However, she suffered from hepatic encephalopathy (disorientation and flapping tremor) in June 2004 and thus was hospitalized. Consequently she and her family decided to undergo living donor liver transplantation at our hospital.
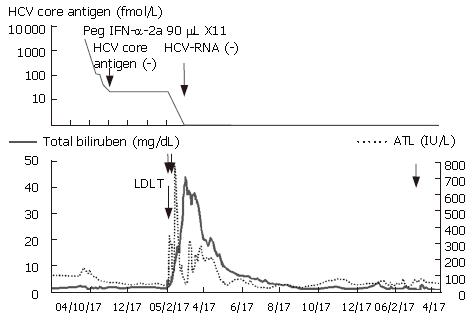
She entered our hospital for evaluation of LDLT in September 2004 (Figure 1). On admission, she had no ascites or hepatic encephalopathy. Laboratory data revealed 2.0 mg/dL total bilirubin, 97 IU/L AST, 88 IU/L ALT, 855 IU/L ALP, 6.7 mg/dL total protein, 3.1 mg/dL albumin, 76% prothrombin time and 81 000/μL platelets, she was thus evaluated to be Child-Pugh grade B. Her HCV genotype was 1b, and the viral load in serum was 1860 KIU/mL by Amplicor PCR or 3320 fmol/L by HCV core antigen assay. HCC remained unchanged on admission. She underwent peg-IFN-α-2a treatment with the goal of virus clearance, because LDLT might be unnecessary depending on the status of her liver function. We concluded that LDLT should be performed after disappearance of HCV-RNA in her serum. She received peg-IFN-α-2a, 90 μg once a week from December 12, but we had to discontinue it due to neutropenia (under 500/μL) after the first week of therapy. As a result, we modified the regimen of peg-IFN-α-2a treatment from once a week to once every two weeks.
At five weeks after initiation of peg-IFN-α-2a treatment, HCV core antigen was not detectable in her serum, but HCV-RNA was still detectable by qualitative PCR(nested PCR) even at 12 wk after initiation of therapy. We therefore decided to perform LDLT with her daughter as donor, since the complete disappearance of HCV-RNA was thought to be impossible. She underwent LDLT on February 18, 2005, and we used peg-IFN-α-2a for the 11th time at 18 wk and HCV-RNA was still positive in the serum at this time.
Surgery was successfully performed. Histopathological examination revealed that the explanted liver exhibited mixed macro and micro nodular cirrhosis, and three tumors were observed in the caudate lobe, one of them showed complete necrosis by TACE while the others were viable and diagnosed with well-differentiated HCC, and another well-differentiated HCC was found in the anterior segment of the explanted liver. There was no-evidence of vascular invasion of HCC. The patient was given tacrolimus and predonisolone as immunosuppressants. On postoperative day (POD) 2, she underwent re-anastomosis of the hepatic artery, because of decreased arterial flow caused by an intimal tear. On POD 5, she underwent the third laparotomy, because of hematoma around the portal vein and thrombosis in the portal vein. Due to food aspiration, methicillin resistance staphylococcus aureus (MRSA) caused pneumonia and a systemic infection. At this time, icterus was worsening and the total bilirubin in serum rose to 30 mg/dL on POD 30. She was treated with intensive care, tracheostomy, artificial respiration, MRSA specific antibiotics, hyperbaric oxygen therapy and steroid pulse therapy. Finally, at post operative month (POM) 4, she was weaned off artificial respiration and her total bililubin decreased to 3.1 mg/dL and she showed a good recovery at POM 8. At POM 13, she was operated on for anastomotic stricture of the common bile duct and was then treated on an out-patient basis.
Serum HCV-RNA was negative at POM 1 and sub-sequently dissolved at POM 15. Liver biopsies performed at POD 2, 5, 32 and POM 13 revealed no findings of HCV reactivation.
Crippin JS and colleagues[9] tried IFN-α-2b (1 or 3 MU/d three time a week) +/- ribavirin (400 mg) within 12 weeks, and a loss of detectable HCV-RNA was seen in 5/15 (33%) patients. Thomas RM and colleagues[10] reported that 12 cases (60%) responded to IFN-α-2b 5 MU/d therapy before LT with a clearance of serum HCV-RNA, four of the 12 cases did not show any evidence of HCV recurrence after LD. Forns X and colleagues[11] used IFN-α-2b 3 MU/d + ribavirin 800 mg/d and 9 cases (30%) demonstrated a clearance of serum HCV-RNA, 6 of the 9 cases did not have any evidence of HCV recurrence after LD. Recently, Everson GT and colleagues[12] described that low dose IFN treatment, peg-IFN-α-2b 0.5 μg/kg per week or IFN-α-2b 1.5 MU/d + ribavirin 600 mg/d per six months for genotypes 2 and 3 or 1 year for genotype 1, was performed for advanced HCV patients, and 12 of 15 cases showing a clearance of HCV-RNA before LT remained HCV-RNA negative 6 or more months after transplantation and 32 cases who were positive for HCV-RNA before remained HCV-RNA positive. In a previous report[13], IFN therapy before LT was shown to be an effective treatment for the clearance of HCV with advanced LC, but sustained HCV clearance after LT was never acquired in patients with a detectable level of HCV before LT. In contrast, our case demonstrated a clearance of HCV-RNA regardless of the fact that HCV-RNA in serum was positive before LDLT. After liver transplantation, we did not prescribe cyclosporine A and mycophenorate mofetil exerting anti-HCV effects in vitro. We hypothesize that peg-IFN-α-2a as a long acting IFN, when injected immediately prior to LDLT, may thus induce an anti-viral activity in the anhepatic phase and also soon after LDLT. The HCV titer is the lowest in the anhepatic phase and the immediately early post LDLT[4], therefore this period requires treatment in order to achieve a clearance of HCV. In our case, HCV showed trace quantities due to the negative HCV core antigen and positive HCV-RNA. We thus speculate that trace quantities of HCV in the graft respond to the continuing presence of peg-IFN-α-2a, though a similar case has not been reported up to now.
In cases of a sustained viral response to IFN therapy after LT, hepatic fibrosis does not progress except for other reasons of hepatic injury, rejection and stenosis of bile[14,15]. HCV relapse post SVR after LT has been reported at 7, 8 and 15 mo after treatment[15,16]. For the reasons stated above, we must pay attention to advanced liver fibrosis and HCV-RNA in the serum.
Based on our findings, we hypothesize that positive HCV-RNA is positive and negative HCV core antigen before LT may lead to a clearance of HCV after LT, and long acting peg-IFN-α-2a may be a potentially effective agent for HCV infection before LT. This hypothesis should be further confirmed.
S- Editor Liu Y L- Editor Wang XL E- Editor Liu Y
| 1. | Takada Y, Ueda M, Ito T, Sakamoto S, Haga H, Maetani Y, Ogawa K, Kasahara M, Oike F, Egawa H. Living donor liver transplantation as a second-line therapeutic strategy for patients with hepatocellular carcinoma. Liver Transpl. 2006;12:912-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Berenguer M, Ferrell L, Watson J, Prieto M, Kim M, Rayón M, Córdoba J, Herola A, Ascher N, Mir J. HCV-related fibrosis progression following liver transplantation: increase in recent years. J Hepatol. 2000;32:673-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 595] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 3. | Terrault NA. Prophylactic and preemptive therapies for hepatitis C virus-infected patients undergoing liver transplantation. Liver Transpl. 2003;9:S95-S100. [PubMed] |
| 4. | Garcia-Retortillo M, Forns X, Feliu A, Moitinho E, Costa J, Navasa M, Rimola A, Rodes J. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology. 2002;35:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 381] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 5. | Powers KA, Ribeiro RM, Patel K, Pianko S, Nyberg L, Pockros P, Conrad AJ, McHutchison J, Perelson AS. Kinetics of hepatitis C virus reinfection after liver transplantation. Liver Transpl. 2006;12:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Shergill AK, Khalili M, Straley S, Bollinger K, Roberts JP, Ascher NA, Terrault NA. Applicability, tolerability and efficacy of preemptive antiviral therapy in hepatitis C-infected patients undergoing liver transplantation. Am J Transplant. 2005;5:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Bruno R, Sacchi P, Ciappina V, Zochetti C, Patruno S, Maiocchi L, Filice G. Viral dynamics and pharmacokinetics of peginterferon alpha-2a and peginterferon alpha-2b in naive patients with chronic hepatitis c: a randomized, controlled study. Antivir Ther. 2004;9:491-497. [PubMed] |
| 8. | Chalasani N, Manzarbeitia C, Ferenci P, Vogel W, Fontana RJ, Voigt M, Riely C, Martin P, Teperman L, Jiao J. Peginterferon alfa-2a for hepatitis C after liver transplantation: two randomized, controlled trials. Hepatology. 2005;41:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 197] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | Crippin JS, McCashland T, Terrault N, Sheiner P, Charlton MR. A pilot study of the tolerability and efficacy of antiviral therapy in hepatitis C virus-infected patients awaiting liver transplantation. Liver Transpl. 2002;8:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Thomas RM, Brems JJ, Guzman-Hartman G, Yong S, Cavaliere P, Van Thiel DH. Infection with chronic hepatitis C virus and liver transplantation: a role for interferon therapy before transplantation. Liver Transpl. 2003;9:905-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Forns X, García-Retortillo M, Serrano T, Feliu A, Suarez F, de la Mata M, García-Valdecasas JC, Navasa M, Rimola A, Rodés J. Antiviral therapy of patients with decompensated cirrhosis to prevent recurrence of hepatitis C after liver transplantation. J Hepatol. 2003;39:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 211] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Everson GT, Trotter J, Forman L, Kugelmas M, Halprin A, Fey B, Ray C. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology. 2005;42:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 13. | Henry SD, Metselaar HJ, Lonsdale RC, Kok A, Haagmans BL, Tilanus HW, van der Laan LJ. Mycophenolic acid inhibits hepatitis C virus replication and acts in synergy with cyclosporin A and interferon-alpha. Gastroenterology. 2006;131:1452-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Abdelmalek MF, Firpi RJ, Soldevila-Pico C, Reed AI, Hemming AW, Liu C, Crawford JM, Davis GL, Nelson DR. Sustained viral response to interferon and ribavirin in liver transplant recipients with recurrent hepatitis C. Liver Transpl. 2004;10:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Bizollon T, Pradat P, Mabrut JY, Chevallier M, Adham M, Radenne S, Souquet JC, Ducerf C, Baulieux J, Zoulim F. Benefit of sustained virological response to combination therapy on graft survival of liver transplanted patients with recurrent chronic hepatitis C. Am J Transplant. 2005;5:1909-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Bizollon T, Ahmed SN, Radenne S, Chevallier M, Chevallier P, Parvaz P, Guichard S, Ducerf C, Baulieux J, Zoulim F. Long term histological improvement and clearance of intrahepatic hepatitis C virus RNA following sustained response to interferon-ribavirin combination therapy in liver transplanted patients with hepatitis C virus recurrence. Gut. 2003;52:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |