Published online Aug 14, 2007. doi: 10.3748/wjg.v13.i30.4085
Revised: May 23, 2007
Accepted: June 4, 2007
Published online: August 14, 2007
AIM: To identify the factors associated with virologic breakthrough and to select a subgroup of patients who respond well to lamivudine without developing virologic breakthrough (VBT).
METHODS: Of 79 patients who had received lamivudine therapy for 9-57 mo, 34 were HBeAg-positive and 45 were HBeAg-negative, 24 developed virologic breakthrough and 55 did not. Clinical and virologic factors were compared between the two groups.
RESULTS: The median duration of therapy was 25 (9-57) mo. Virologic breakthrough was defined as a > 1 log HBV DNA increase following initial suppression. When several factors, including gender, duration of infection, baseline HBV DNA, and baseline ALT in HBeAg-positive chronic hepatitis patients were analyzed by logistic regression, the most important predictor of virologic breakthrough was the baseline HBV DNA (r2 = 0.12, P < 0.05). When HBeAg-postitive chronic hepatitis patients were divided into two groups by a point of 6.6 log HBV DNA, the incidence of virologic breakthough between two groups was significantly different.
CONCLUSION: Lamivudine may remain an effective first line therapy for those HBeAg-positive patients with a baseline HBV DNA < 6.6 log10 copies/mL.
- Citation: Chae HB, Hann HW. Baseline HBV DNA level is the most important factor associated with virologic breakthrough in chronic hepatitis B treated with lamivudine. World J Gastroenterol 2007; 13(30): 4085-4090
- URL: https://www.wjgnet.com/1007-9327/full/v13/i30/4085.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i30.4085
A high incidence of virologic breakthrough (VBT) that results from viral resistance is a major disadvantage of lamivudine (LAM) treatment for patients with chronic hepatitis B (CHB). Data from a study of 592 patients who had undergone four years of LAM treatment indicated that resistance increased from 23% in year 1 to 71% in year 4[1,2]. The clinical consequences of viral resistance can be severe, but early studies suggest that median HBV DNA and ALT levels continue to improve even as resistance develops[3]. LAM is known to have long-term therapeutic effects, with well-known resistance and side effects. LAM therapy for CHB patients with advanced fibrosis significantly reduced the incidence of hepatic decompensation and hepatocellular carcinoma (HCC)[4]. In addition, it is becoming increasingly clear that CHB management requires a long-term therapy, thus making the cost of treatment an important consideration. The need for long-term therapy and the consequent financial burden become significant in the economically disadvantaged regions of the world which also happen to be the hyper-endemic areas for HBV. Furthermore, this cost issue presents a serious economic burden for CHB patients who immigrated to the US from the hyper-endemic regions. One study suggested that upfront LAM therapy may be highly cost-effective, assuming that patients who develop resistance begin a regimen of adefovir instead of continuing LAM treatment[5]. Our study sought to identify the factors associated with VBT in patients on LAM therapy and attempted to select a subgroup of patients who may respond well to LAM without developing VBT.
We reviewed the medical records of 208 CHB patients who were treated at the Liver Disease Prevention Center, Division of Gastroenterolgoy and Hepatology at Thomas Jefferson University Hospital, Philadelphia from January 2000 to December 2004. Ninety-seven patients were excluded because they were treated with other anti-viral agents (excluding interferon) prior to their first visits. Five were excluded due to co-infection with Hepatitis C virus. We selected individuals from the remaining 106 patients who had been on LAM therapy for at least 9 mo (which excluded 14 patients) and whose pre-treatment HBV DNA was greater than 3 log10 copies/mL (which excluded 5 patients). Baseline HBeAg status was not available for four patients. Four patients who had suboptimal responses during the entire treatment period were also excluded from the final analysis because of uncertainty of classifying these patients into either the breakthrough or the non-breakthrough group. In the end, clinical and laboratory data from a total of 79 patients were reviewed.
The inclusion criteria consisted of the following two conditions: (1) Patients had received LAM therapy for at least 9 mo; and (2) they had a baseline HBV DNA level ≥ 3 log10 copies/mL. Patients were excluded if they had previously received anti-HBV therapy (with the exception of interferon therapy), or if they had hepatitis C virus or hepatitis D virus co-infection.
All patients had been positive for hepatitis B surface antigen (HBsAg) for more than 6 mo. HBeAg/anti-HBe and anti-HDV were determined using ELISA (Abbott Laboratories, Chicago, IL), and anti-HCV antibodies were assayed using a third-generation enzyme immunoassay (Abbott Laboratories, North Chicago, IL). Between 2000 and 2002, a solution hybridization assay (Abbott Laboratories, North Chicago, IL) with a lower limit of detection (LLOD) of 1.6 pg/mL was used to measure HBV DNA levels. These values were converted to copies/mL by determining 283 000 copies/mL per 1 pg/mL of HBV DNA. From 2003 until the present, HBV DNA was measured by RT-PCR (Quest Diagnostics, Horsham, PA) with a lower limit of detection of 500 copies/mL. Serial dilutions were performed for samples exceeding 5.3 log10 copies/mL. Values below this cutoff were assigned a value of 1 log10 copies/mL.
The initial virologic response was defined as HBV DNA that was less than 4 log10 copies/mL after 6 mo on therapy, and viral suppression was defined as the difference between the level of HBV DNA at baseline and after 6 mo of treatment. Maximal virologic suppression was defined as the difference in HBV DNA levels between baseline and nadir. VBT was defined as > 1 log10 copies increase in HBV DNA from nadir on two consecutive occasions after an initial virologic response or HBV DNA could be detected again after the previous report of “under the detection limit”. Suboptimal responders were defined as patients whose initial virologic response was less than 2 log during the entire period of treatment.
HBV DNA was extracted using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The presence of HBV DNA polymerase gene mutations was determined using the InnoLiPA DR2 line probe assay (InnoGenetics, Ghent, Belgium) according to the manufacturer’s instructions. Line probe results were confirmed by bi-directional automated sequencing at the DNA sequencing core facility (Thomas Jefferson University’s nucleic acid facility, Philadelphia, PA) using the standard protocol for the Applied Biosystems DNA Sequencer 377 (Perkin Elmer Corp., Foster City, CA). This amplicon covers domains A, B, C, D, and E of the reverse transcriptase region of the HBV polymerase. The PCR protocol and primers for the surface/polymerase gene and core promotor/precore region used in this study were described previously[6].
Statistical testing was performed using SPSS version 13.0 (SPSS Inc., Chicago IL). Results were expressed as mean ± SD or median (minimum-maximum). HBV DNA levels were logarithmically transformed for analysis. Continuous variables were compared using the two-tailed student’s t-test, and categorical data were compared using the two-tailed χ2 test. Factors associated with an initial virologic response and LAM resistance were analyzed by univariate analysis. Clinical, biochemical and virologic factors that could influence LAM resistance, including gender, route of infection, baseline ALT level, baseline HBV DNA level, were analyzed by binary logistic regression. The cumulative probability of lamivudine resistance was estimated by Kaplan-Meier analysis. P < 0.05 was considered statistically significant.
Seventy-nine patients (54 males) met eligibility criteria. All were Asian Americans, with a mean age of 47 ± 12 years. Thirty-seven (45%) had been infected at birth by vertical transmission from their mothers. Twenty-four patients (30%) had liver cirrhosis and two (2%) had liver cancer prior to starting LAM therapy. The mean duration of therapy was 26 ± 10 mo. At baseline, the ALT was 66 (11-3395) IU/L and the mean log HBV DNA level was 6.0 ± 1.6. The baseline HBV DNA level in the 34 HBeAg-positive patients was 6.6 ± 1.5, and the baseline DNA level in the 45 HBeAg-negative patients was 5.6 ± 1.5 log10 copies/mL. Treatment was initiated if HBV DNA levels were ≥ 3 log10 copies/mL. Eighteen patients had HBV DNA levels between 3 and 5 log10 copies/mL. Based on earlier observations of 317 CHB patients[7], normal pre-therapy ALT was not considered a contraindication for beginning antiviral therapy in certain patients. These included patients who had normal ALT levels, high viral DNA, and a strong family history of HCC or patients who had had previous ALT spikes but whose ALT levels were normal at the beginning of the study. All baseline characteristics except HBV DNA level were similar between the VBT and non-VBT groups. Only ALT level was shown as mean ± SE (Table 1).
| All patients(n = 79) | Breakthrough(n = 24) | Non-breakthrough(n = 55) | |
| Age (yr) | 47 ± 12 | 47 ± 13 | 47 ± 11 |
| Female (%) | 32 | 29 | 33 |
| Family history of CHB (%) | 47 | 46 | 55 |
| ALT (IU/L) | 149 ± 44 | 98 ± 20 | 171 ± 63 |
| Total bilirubin (mg/dL) | 1.1 ± 1.1 | 1.3 ± 1.4 | 1.0 ± 0.9 |
| Albumin (g/dL) | 4.4 ± 0.4 | 4.2 ± 0.5 | 4.3 ± 0.4 |
| (INR) | 1.07 ± 0.12 | 1.09 ± 0.15 | 1.06 ± 0.08 |
| Cirrhosis (%) | 30 | 36 | 24 |
| HBeAg-positive (%) | 43 | 56 | 36 |
| HBV DNA log10 copies/mLa | 6.0 ± 1.6 | 6.6 ± 1.5 | 5.7 ± 1.5 |
| Mean duration of lamivudine treatment (mo) | 26 ± 10 | 29 ± 11 | 25 ± 10 |
| 3 log < HBV DNA < 5 log (%) | 19 | 3 | 16 |
| HBV DNA ≥ 5 log (%) | 60 | 21 | 39 |
In HBeAg-positive patients (n = 34), the mean log reduction (copies/mL) in HBV DNA from baseline was 4.6 ± 1.4, 3.7 ± 2.4 and 2.7 ± 3.0 at 6 mo, 12 mo and 24 mo, respectively. The less reduction in two years may be due to VBT in some patients. As shown in Table 2, initial viral suppression was 4.2 ± 2.0 and 4.3 ± 1.5 in VBT and non-VBT groups, respectively. The maximal viral suppression was 4.8 ± 1.7 and 4.6 ± 1.2 in VBT and non-VBT groups, respectively. Eighty percent of patients showed an initial anti-viral response at 6 mo post-treatment. HBeAg loss rate was 18, 26, and 29% at 1, 2, and 3 years, respectively. Excluding individuals with normal or absent ALT levels at baseline, ALT normalization was observed in 90% and 96% of patients after 6 mo and 12 mo, respectively. The mean time for VBT detection was 21 (9-36) mo. There was no viral or biochemical variable with a significant difference between VBT and non-VBT groups.
| HBeAg-positive hepatitis (n = 34) | HbeAg-negative hepatitis (n = 45) | |||||
| All patients(n = 34) | BT(n = 14) | No BT(n = 20) | All patients(n = 45) | BT(n = 10) | No BT(n = 35) | |
| Max suppression (log10 copies/mL) | 4.7 ± 1.4 | 4.8 ± 1.7 | 4.6 ± 1.2 | 4.3 ± 1.6 | 4.3 ± 1.7 | 4.3 ± 1.6 |
| Initial suppression (log10 copies/mL) | 4.2 ± 1.7 | 4.2 ± 2.0 | 4.3 ± 1.5 | 3.3 ± 2.1 | 2.8 ± 2.5 | 3.4 ± 1.9 |
| Patients with IVR (%) | 80 | 75 | 83 | 81 | 67 | 85 |
| Patients at 6 mo biochemical response (%) | 90 | 82 | 94 | 84 | 86 | 83 |
| Patients at 12 mo biochemical response (%) | 96 | 100 | 93 | 82 | 80 | 82 |
| Time to first detection of BT | - | 26 ± 10 | - | - | 32 ± 12 | - |
In HBeAg-negative patients (n = 45), the mean log reduction (copies/mL) in HBV DNA from baseline was 3.2 ± 2.0 at 6 mo, 4.2 ± 1.9 at one year, and 3.1 ± 3.0 at two years. Initial viral suppression was 2.8 ± 2.5 and 3.4 ± 1.9 in VBT and non-VBT groups, respectively. Maximal suppression was 4.3 ± 1.7 and 4.3 ± 1.6 in VBT and non-VBT groups, respectively. Eighty-one percent of patients showed an initial antiviral response at 6 mo post-treatment. Excluding individuals with normal or absent ALT levels at baseline, ALT normalization was observed in 84% and 82% of patients after 6 mo and 12 mo, respectively. The mean time for VBT detection was 22 (9-36) mo. Also, there was no viral or biochemical variable with a significant difference between VBT and non-VBT groups.
HBV DNA sequences were examined in nine patients’ sera that were collected at the time of baseline and VBT. Six patients without VBT were also examined as controls. The samples from 15 patients were investigated for genotype, precore mutant, and core promoter mutant. As shown in Table 3, YMDD mutants were observed in six of the nine individuals in the VBT group and none were observed in the non-VBT group (67% vs 0%, P < 0.01). All patients had HBV genotype C. The frequency of precore and core promotor variants did not differ significantly between VBT and non-VBT groups.
| Patients with VBT | During BT | ||||||||||
| Patient | Sex | BT | Genotype | Month after LAM | ALT | HBeAg | HBV DNA log10 copies/mL | PC | CP A1763T G1765A | L180M | M204I/V |
| 1 | F | + | C | 19 | 90 | - | 5.4 | Wild | TA | M | I/V |
| 2 | M | + | C | 18 | 29 | - | 3 | Stop | TA | L | I/V |
| 3 | F | + | C | 12 | 12 | + | 6.8 | Wild | AG | L | M |
| 4 | M | + | C | 18 | 44 | + | 8.3 | Wild | TA | M | I/V |
| 5 | M | + | C | 36 | NA | + | 5.2 | Wild | TA | M | I/V |
| 6 | F | + | C | 21 | 42 | + | 5.7 | Wild | AG | L | M |
| 7 | M | + | C | 27 | 24 | + | 6.6 | Stop | TA | M | I/V |
| 8 | F | + | C | 21 | NA | + | 6.7 | Wild | AG | L | M |
| 9 | F | + | C | 30 | 108 | + | 6.7 | Wild | TA | M | I/V |
| Patients without VBT | End of follow-up | ||||||||||
| Patient | Sex | BT | Genotype | Month after LAM | ALT | HBeAg | HBV DNA log10 copies/mL | PC | CP A1763T G1765A | L180M | M204I/V |
| 1 | M | - | C | 9 | 28 | + | 4.6 | Wild | TA | L | M |
| 2 | M | - | C | 30 | NA | + | 5.4 | Wild | TA | L | M |
| 3 | M | - | C | 32 | 12 | - | 1 | UD | UD | L | M |
| 4 | M | - | C | 27 | NA | + | 1 | Wild | TA | L | M |
| 5 | M | - | C | 36 | 42 | - | 1 | UD | UD | L | M |
| 6 | M | - | C | 24 | 24 | + | 1 | Wild | TA | UD | UD |
Patient 6 in the VBT group had a precore mutation in the 1896 position and was HBeAg-positive (Table 3). Patients 3 and 8 also had smaller A peaks than G peaks at the 1896 position, but were classified as wild-type (Table 3).
When all factors, including gender, duration of infection, baseline HBV DNA, HBV DNA at the 6th month of treatment, and baseline ALT in HBeAg-positive CHB patients were analyzed by logistic regression, the most important factor associated with VBT was only baseline HBV DNA level (r2 = 0.12, P < 0.05) .
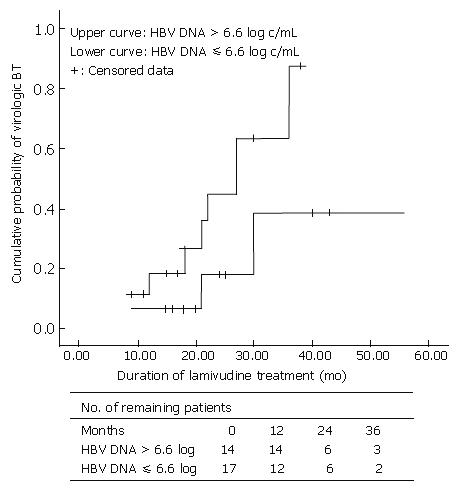
When HBeAg-positive CHB patients were divided into two groups by the point of 6.6 log HBV DNA level: one group > 6.6 log HBV DNA; and other group ≤ 6.6 log HBV DNA, there was a significant difference between two groups in terms of VBT rate (P < 0.05). We also compared the cumulative VBT rate between these two groups according to the treatment duration with Kaplan-Meier analysis. The VBT percentage in HBeAg-positive hepatitis patients with HBV DNA levels ≤ 6.6 log10 (n = 15) was 6.7%, 18%, and 39% at 1, 2, and 3 years, respectively, while that in patients with HBV DNA levels > 6.6 log10 (n = 17) was 19%, 45%, and 88% at 1, 2, and 3 years, respectively (P = 0.061) (Figure 1).
The next important factor associated with VBT was HBV DNA at the 6th month of treatment, but it did not show a significant correlation with VBT. In HBeAg-negative patients, no relationship between the VBT rate and HBV DNA level was observed.
Our study demonstrated that patients with lower HBV DNA levels had less VBT with LAM therapy. Other predictors of LAM-resistant mutations include male gender, Asian ethnicity, higher baseline HBV DNA, longer duration of lamivudine treatment, and higher BMI[2]. Our study clearly showed that the baseline HBV DNA level is the most significant predictor. However, it is true that the number of study subjects was relatively small (n = 79) and the median duration of follow-up was about 40 mo.
But, the findings of our study are contradictory to the observation of the Taiwanese study[8]. They reported that HBeAg status, HBV DNA, ALT levels and treatment duration were the major determinants for the YMDD mutation during lamivudine therapy. The most important difference between two studies is what they observed. We observed VBT, but they observed the genotypic resistance. Besides, there were several differences between two studies, such as study designs and the test for HBV DNA.
When the study criteria were set up, AGA (American Gastroenterology Association) expert panel recommendations were followed; HBeAg-positive patients were individuals with HBV DNA > 5 log10 copies/mL, while HBeAg-negative patients were individuals with HBV DNA > 4 log10 copies/mL. In this study, a lower HBV DNA cutoff, above 3 log, was used for starting antiviral treatment. Although we did not know the recent Taiwanese data when we started to treat our patients, but it shows that higher baseline serum DNA levels are associated with increased risk of HCC and liver cirrhosis independent of serum ALT level[9]. The AGA panel specifies that ALT levels should be abnormal, but this is not always helpful in determining who should be treated[10]. ALT levels do not serve as a good indicator of liver cirrhosis. In addition, revision of the normal limits for ALT levels was recommended in patients with chronic HCV infection or nonalcoholic fatty liver disease (NAFLD) since current standards for normal were defined using the populations that included individuals with subclinical disease[11,12]. Some patients experienced disease progression during follow-up because they had normal ALT levels and were not treated earlier. Thus, early antiviral treatment may be beneficial to reduce HBV DNA levels, HCC risk, and the frequency of liver transplantations[13]. LAM can reduce serum HBV DNA levels and normalize ALT level in cirrhotic patients[14,15].
As shown in Table 1, we noted that HBeAg-negative CHB have lesser LAM resistance than HBeAg-positive CHB, which is in agreement with previous studies[16,17].
We conducted genotypic resistance analysis on 15 patients who had serum samples harvested upon viral breakthrough or at the end of follow-up. Genotypic resistance occurred only in the VBT group and was not observed in the non-VBT group. However, three patients in the VBT group, and five in the non-VBT group had the wild-type. The most plausible explanation for the disparity between phenotypic and genotypic resistance is medication noncompliance. Possible solutions for this problem, like pill counts and nurse monitoring, were beyond the scope of our study. A blip in viral numbers is another potential explanation for the presence of wild-type virus in the VBT patients. The InnoLiPa assay could be used to define the mixed strains in six patients from the VBT group. When we found the viral breakthrough in 25 patients, we recommended several options such as adefovir monotherapy, adefovir overlapped with lamivudine, and tenofovir monotherapy to our patients.
Two patients of suboptimal responders had YMDD mutants. They did not show VBT during treatment period. But we might have missed VBT in these patients because the test for HBV DNA had not been tested at the right time, or YMDD mutants may have existed even before LAM monotherapy[18].
The experimental results from both direct sequencing and InnoLipa DR2 line probe assay were consistent in all patients.
The monthly U.S. wholesale prices for hepatitis B treatment are $158 for lamivudine (100 mg/d), $528 for adefovir (10 mg/d), $715 for entecavir (0.5 mg/d), $1429 for entecavir salvage therapy (1 mg/d), and $1540 for peginterferon alpha-2a (180 μg/wk). Thus, the most cost-effective regimen across most healthcare settings, independent of HBeAg status, is LAM monotherapy, and adefovir salvage therapy for individuals with LAM resistance[4].
Our study was limited by the small patient sample size as well as potential referral bias. We were unable to conduct genotypic resistance analysis for all subjects since serum samples were not always available.
We observed the correlation between the occurrence of VBT and initial HBV DNA level. We suggest that 6.6 log10 in HBeAg-positive CHB patients can be a criterion for long-term LAM monotherapy and the patients with baseline HBV DNA level ≤ 6.6 log may derive the most benefit from LAM monotherapy because of low incidence of VBT. For individuals with CHB, who have a baseline HBV DNA ≤ 6.6 log and limited financial means, we conclude that LAM remains an affordable and effective therapy.
Dr. Hann is on the speaker’s bureau for Gilead, Bristol-Myers Squibb and GlaxoSmithKline, and has received research support from all three companies The authors are grateful to Dr. Scott Fung at the University of Toronto for his valuable advice in preparing the manuscript, Ms. Munira Hussain at the University of Michigan for providing invaluable information about primers and the PCR protocol, and Dr. Mark Feitelson for providing the laboratory facility to conduct the molecular biology.
Lamivudine is the first FDA-approved oral antiviral agents for chronic hepatitis B (CHB) with 10 years experience of safety and efficacy. It was recently excluded from the 1st line drugs in 2007 AASLD practice guideline because of its high rate of drug resistance. We investigated the factors associated with virologic breakthrough in patients on lamivudine therapy and identified a subgroup of patients who responded well to lamivudine without developing virologic breakthrough. Therefore, we believe that for this subgroup of CHB patients, lamivudine may still remain the most cost-effective drug among 4 currently available oral anti-HBV drugs.
Other predictors of lamivudine resistance include male gender, Asian ethnicity, higher baseline HBV DNA, long duration of lamivudine treatment, and higher BMI.
Our study clearly showed that the baseline HBV DNA level is the most significant predictor for virologic breakthrough and identified the discriminating number as 6.6 log10 HBV DNA.
Physicians can apply this knowledge to predict lamivudine resistance and may start lamivudine for this group of patients who present with baseline HBV DNA level lower than 6.6 log.
Virologic breakthrough: > 1 log10 copies increase in HBV DNA from nadir on two consecutive occasions after an initial virologic response.
The authors investigated several clinical, biochemical and virologic factors, including gender, route of infection, duration of infection, baseline HBV DNA level, baseline ALT level, and other factors in HBeAg-positive chronic hepatitis B patients. The study is well conducted and the results indicate that the most important predictor of virologic breakthrough is the baseline HBV DNA.
S- Editor Zhu LH L- Editor Kumar M E- Editor Wang HF
| 1. | Chang TT, Lai CL, Chien RN, Guan R, Lim SG, Lee CM, Ng KY, Nicholls GJ, Dent JC, Leung NW. Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2004;19:1276-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, Dienstag JL, Heathcote EJ, Little NR, Griffiths DA. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 589] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 3. | Lai CL, Dienstag J, Schiff E, Leung NW, Atkins M, Hunt C, Brown N, Woessner M, Boehme R, Condreay L. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis. 2003;36:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 493] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 4. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1740] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 5. | Kanwal F, Gralnek IM, Hays RD, Dulai GS, Spiegel BM, Bozzette S, Asch S. Impact of chronic viral hepatitis on health-related quality of life in HIV: results from a nationally representative sample. Am J Gastroenterol. 2005;100:1984-1994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Lok AS, Zoulim F, Locarnini S, Mangia A, Niro G, Decraemer H, Maertens G, Hulstaert F, De Vreese K, Sablon E. Monitoring drug resistance in chronic hepatitis B virus (HBV)-infected patients during lamivudine therapy: evaluation of performance of INNO-LiPA HBV DR assay. J Clin Microbiol. 2002;40:3729-3734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Hann HW, Jonsson Funk ML, Rosenberg DM, Davis R. Factors associated with response to lamivudine: Retrospective study in a tertiary care clinic serving patients with chronic hepatitis B. J Gastroenterol Hepatol. 2005;20:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Chang ML, Chien RN, Yeh CT, Liaw YF. Virus and transaminase levels determine the emergence of drug resistance during long-term lamivudine therapy in chronic hepatitis B. J Hepatol. 2005;43:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2309] [Cited by in RCA: 2365] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 10. | Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, Tobias H, Wright TL. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States. Clin Gastroenterol Hepatol. 2004;2:87-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Prati D, Rebulla P, Zanella A, Fraquelli M, Conte D. Peripheral blood count abnormalities among patients with hepatitis C in the United States. Hepatology. 2002;36:1025-1026; author reply 1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Yuen MF, Yuan HJ, Wong DK, Yuen JC, Wong WM, Chan AO, Wong BC, Lai KC, Lai CL. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut. 2005;54:1610-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 292] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 13. | Perrillo RP, Wright T, Rakela J, Levy G, Schiff E, Gish R, Martin P, Dienstag J, Adams P, Dickson R. A multicenter United States-Canadian trial to assess lamivudine monotherapy before and after liver transplantation for chronic hepatitis B. Hepatology. 2001;33:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 310] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 14. | Villeneuve JP, Condreay LD, Willems B, Pomier-Layrargues G, Fenyves D, Bilodeau M, Leduc R, Peltekian K, Wong F, Margulies M. Lamivudine treatment for decompensated cirrhosis resulting from chronic hepatitis B. Hepatology. 2000;31:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 295] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 15. | Hann HW, Fontana RJ, Wright T, Everson G, Baker A, Schiff ER, Riely C, Anschuetz G, Gardner SD, Brown N. A United States compassionate use study of lamivudine treatment in nontransplantation candidates with decompensated hepatitis B virus-related cirrhosis. Liver Transpl. 2003;9:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1107] [Cited by in RCA: 1090] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 17. | Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 915] [Cited by in RCA: 910] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 18. | Paik YH, Chung HY, Ryu WS, Lee KS, Lee JS, Kim JH, Lee CK, Chon CY, Moon YM, Han KH. Emergence of YMDD motif mutant of hepatitis B virus during short-term lamivudine therapy in South Korea. J Hepatol. 2001;35:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |