Published online Aug 14, 2007. doi: 10.3748/wjg.v13.i30.4072
Revised: December 23, 2006
Accepted: January 1, 2007
Published online: August 14, 2007
AIM: To compare the antiviral efficacy of adefovir (ADV) in lamivudine (LMV)-resistant patients with LMV treatment in nucleoside-naïve patients, using serum samples collected sequentially during the course of treatment progressing from LMV to ADV.
METHODS: Forty-four patients with chronic hepatitis B (CHB) were included. The patients were initially treated with LMV and then switched to ADV when LMV resistance developed. Antiviral efficacy was assessed by measuring the following: reduction in serum HBV DNA from baseline, HBV DNA negative conversion (defined as HBV DNA being undectable by the hybridization assay), and HBV DNA response (either HBV DNA level ≤ 105 copies/mL or a ≥ 2 log10 reduction from baseline HBV DNA level).
RESULTS: After two and six months of treatment, HBV DNA reduction was greater with LMV compared to ADV treatment (P = 0.021). HBV DNA negative conversion rates were 64% and 27% after one month of LMV and ADV treatment respectively (P = 0.001). Similarly, HBV DNA response rates were 74% and 51% after two months of LMV and ADV treatment respectively (P = 0.026).
The time taken to HBV DNA negative conversion and to HBV DNA response were both delayed in ADV treatment compared with LMV.
CONCLUSION: The antiviral efficacy of ADV in LMV-resistant patients is slower and less potent than that with LMV in nucleoside-naïve patients during the early course of treatment.
- Citation: Seo YS, Kim JH, Yeon JE, Park JJ, Kim JS, Byun KS, Bak YT, Lee CH. Antiviral efficacy of adefovir dipivoxil versus lamivudine in patients with chronic hepatitis B sequentially treated with lamivudine and adefovir due to lamivudine resistance. World J Gastroenterol 2007; 13(30): 4072-4079
- URL: https://www.wjgnet.com/1007-9327/full/v13/i30/4072.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i30.4072
Chronic hepatitis B (CHB) is a common disease, with an estimated prevalence of approximately 5% of the world’s population[1]. Carriers of hepatitis B virus (HBV) are at an increased risk of developing cirrhosis, hepatic decompensation, and hepatocellular carcinoma[2], and these complications result in greater than 1 million deaths annually[3]. Therefore, the ultimate goal of therapy is to limit or reverse the progression of the disease by sustained suppression of HBV replication[4]. This goal can be achieved with the use of well-tolerated antiviral agents that provide clinical benefit without inducing resistance. Lamivudine (LMV) and adefovir dipivoxil (ADV) are safe and efficacious drugs licensed for the treatment of CHB. LMV was the first oral drug licensed for the treatment of CHB. It increases hepatitis B e antigen (HBeAg) seroconversion, improves HBV-associated liver disease and reduces the progression of hepatic fibrosis, and the development of cirrhosis[5-8]. However, selective amplification of LMV-resistant mutants is the main concern with long-term LMV treatment[9,10]. The prevalence of resistant mutants is 16%-32% during the first year of treatment and increases by approximately 15% with each year of additional treatment[11-14]. Emergence of LMV-resistance has been reported to be associated with diminished clinical and virological response to LMV[6,15,16]. Exacerbation of CHB was reported to develop in 40.6% patients carrying LMV-resistant mutants during continued LMV treatment[17]. LMV-resistance is associated with advanced hepatic fibrosis and severe microinflammatory changes in patients with recurrent HBV infection after liver transplantation[18]. Furthermore, hepatic decompensation and death can occur, particularly in patients with cirrhosis[17,19-22]. In addition, the risk of hepatocellular carcinoma may be increased in patients with LMV-resistance[23]. Therefore, management of LMV-resistant mutants is a major concern in clinical practice.
ADV use is associated with a low incidence of viral resistance[24-26] and this drug has potent antiviral efficacy in nucleoside-naïve patients with CHB, resulting in significant biochemical, virological, and histological improvement. Moreover, ADV is efficacious against LMV-resistant HBV[27-29]. With an increasing number of patients undergoing prolonged LMV treatment, the potential candidates for ADV is growing. However, there are no reports on a direct comparison between the use of ADV for treating patients with LMV-resistant hepatitis B, and the use of LMV for treating nucleoside-naïve patients, in terms of antiviral efficacy and the duration of treatment required.
The present study was carried out to compare the antiviral efficacy of ADV in patients with LMV-resistant strains and LMV in nucleoside-naïve states, using serum samples collected sequentially from 44 patients with CHB during the course of progression from LMV to ADV treatment.
Data was collected retrospectively from 44 patients treated with LMV initially and switched to ADV because of development of LMV-resistant HBV infection. Serum samples were obtained at baseline and 1, 2, 3, 6, and 12 mo after commencement of LMV and ADV treatment and kept at -70°C until HBV DNA levels were measured by real time PCR. All patients were negative for antibodies to human immunodeficiency virus and hepatitis C.
Tests for biochemical liver-functions and viral replication, including HBeAg, anti-HBe antibodies and HBV DNA levels, were assessed every 1-3 mo during the treatment period. The HBV DNA levels were quantified using both the hybridization technique (HBV Test, Hybrid Capture II, Digene Corp., Gaithersburg, MD; detection limit, 0.5 pg/mL) and real time PCR assay (GeneMatrix Inc, Seoul, Korea; detection range, 366-3.66 × 1011 copies/mL).
The protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by Korea University Guro Hospital human research committee.
Antiviral treatment efficacy was determined by the reduction in HBV DNA levels from baseline, HBeAg seroconversion, HBV DNA negative conversion, and HBV DNA response. HBeAg seroconversion was defined as the loss of HBeAg and detection of anti-HBe antibodies in patients whose baseline HBeAg was positive. HBV DNA negative conversion was defined as loss of HBV DNA determined by the hybridization assay on two or more consecutive occasions, at least three months apart. HBV DNA response was defined as HBV DNA level ≤ 105 copies/mL or a ≥ 2 log10 reduction from baseline HBV DNA level[33]. Viral breakthrough (V-BT) was defined as the reappearance of HBV DNA, measured by the hybridization assay, in patients whose level had become undetectable for at least three months after commencement of antiviral treatment.
Quantitative analysis of serum HBV DNA was performed retrospectively from stored serum samples. Viral DNA was extracted using Qiagen Blood Kits (Qiagen, Chatworth, CA, USA) according to the manufacturer’s instructions. PCR amplifications were performed using a 25 μL reaction mix containing 300 nmol/L of the forward and 900 nmol/L reverse primers, and 250 nmol/L TaqMan probe (Perkin Elmer Biosystems, Foster City, CA, USA), TaqMan universal PCR masterMix (Applied Biosystems, Foster City, CA, USA) and 5 μL HBV DNA. An ABI prism model 7300 (Applied Biosystems) continuously detected amplified signals. The following real time PCR amplification protocol was used: (1) initial minimal re-amplification of carry over product with uracil-N-glycosylase (AmpErase, Applied Biosystems) at 50°C for 10 min, and (2) a double round of amplification and quantification involving: 45 cycles at 95°C for 15 s and at 60°C for 60 s. The respective sequences of forward primer, reverse primer and TaqMan probe were as follows: 5’-CCgTCTgTgCCTTCTCATCTg-3’ (HBV1F, nucleiotides 1549-1569), 5’-AgTCCAAgAgTTCTCTTATg YAAgACCTT-3’ (HBV1R, nucleotides 1641-1669), and 5’ FAM-CCgTgTgCACTTCgCTTCACCTCTgC-TAMRA 3’ (HBV1TAQ, nucleotides 1575-1600). Nucleotide sequence positions were numbered according to Ono et al[31].
The absolute amount of HBV DNA was quantified using a standard curve generated from subcloned pUC119 (Takara, Japan), a recombinant plasmid containing the entire 3.2 kb of HBV DNA. The linear dynamic range of detection was 366-3.66 × 1011 copies/mL.
Aliquots of 2 μL of viral DNA were used for PCR reactions. For genotyping, matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS; Bruker Daltonics Biflex IV, Billerica, MA, USA), termed RFMP, PCR was performed in 18 μL of reaction mixture containing 20 mmol/L Tris-HCl (pH 8.4), 50 mmol/L KCl, 0.2 mmol/L of each dNTP, 10 pmoL of each primer, and 0.4 units of Platinum® Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA). The amplification conditions included initial denaturation at 94°C for 2 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s. The sequences of forward and reverse primers used in the PCR were respectively: 5’-TTCCCCCACTgTTTggCTggATgTCAgTTAT-3’ (nucleotide numbers 712-738) and 5’-TACAgACTTggCCCCCAATACCACATgA-3’ (nucleotide numbers 771-744). To insert a new FokI digestion site or to eliminate the naturally occurring FokI site in the products, sequences underlined in each primer were modified as described in Hong et al[32]. PCR was performed as above to amplify the HBV polymerase gene encoding the YMDD motif for cloning or for sequencing analyses. Nucleotide sequence positions were numbered according to Ono et al[31].
Restriction enzyme digestion of PCR products was performed by mixing the PCR reaction mixture with 10 μL of buffer containing 50 mmol/L potassium acetate, 20 mmol/L Tris-acetate, 10 mmol/L magnesium acetate, 1 mmol/L dithiothreitol and 1 unit of FokI. The reaction mixture was incubated at 37°C for 2 h and further incubated at 45°C for 2 h with BstF5I. The resulting digest was desalted by vacuum filtration through a 384-well sample preparation plate containing 5 mg of polymeric solvent (Waters, Miliford, MA, USA) per well. The desalted reaction mixtures were resuspended with matrix solution containing 50 mg/mL 3-hydroxy picolinic acid, 0.05 mol/L ammonium citrate and 30% acetonitrile, and were spotted in 3 μL volumes on a polished anchorchip plate. Mass spectra were acquired on linear Bruker Daltonics MALDI-TOF MS workstation in a positive ion, delayed extraction mode.
The data was analyzed using the statistical package SPSS (version 10.0; SPSS Inc., Chicago, IL, USA). The results are expressed as the means ± standard deviations (SD). HBV DNA levels are expressed as logarithmic scales. Quantitative values are expressed as means and ranges, and were compared using the Student’s t-test or the Mann-Whitney nonparametric U test. Kaplan-Meier estimates and log-rank analyses were used to identify factors associated with the time to HBeAg seroconversion. Qualitative values were correlated with Chi-square or Fisher exact tests. In all cases, P < 0.05 (two-tailed) was considered statistically significant.
Table 1 summarizes the baseline characteristics of 44 patients with CHB sequentially treated with LMV and ADV. The mean age was 45 ± 11.2 years (range, 17-67). Thirty patients were men and 13 had cirrhosis. LMV treatment was given for a mean of 29 ± 15.4 mo (range, 7-68). During LMV treatment, cirrhosis developed in five additional patients. V-BT emerged at a mean of 17 ± 8.5 mo of LMV treatment (range, 5-44 mo).
| Lamivudine | Adefovir | P | |
| ALT level (IU/L) | 310 ± 251 | 336 ± 379 | NS |
| Total bilirubin level (mg/dL) | 1.7 ± 2.1 | 1.5 ± 1.0 | NS |
| Albumin level (g/dL) | 3.8 ± 0.6 | 3.9 ± 0.7 | NS |
| Positive for HBeAg [Nos. (%)] | 39 (88.6) | 33 (75.0) | NS |
| HBV DNA level (log copies/mL)1 | 7.64 ± 0.77 | 7.36 ± 1.16 | NS |
Because of the development of LMV-resistant HBV, all patients were treated with ADV. In 17 patients, LMV was maintained for the initial 1-4 mo (median, 3 mo) of ADV treatment and in 17 patients, LMV was discontinued with the commencement of ADV treatment. The remaining 10 patients were treated with ADV after a treatment-free period of 5 mo (median, range, 1-15 mo).
The baseline serum alanine aminotransferase (ALT), total bilirubin and albumin levels and HBeAg status were not significantly different between the LMV and ADV treatment groups. The mean baseline HBV DNA levels were 7.64 and 7.36 log10 copies/mL respectively.
The different types of LMV-resistance mutations are summarized in Table 2. This analysis was performed in 40 of the 44 study patients; in remaining four, LMV-resistance was diagnosed clinically by the reappearance of HBV DNA (assessed by hybridization assay) after initial HBV DNA negative conversion. The most common LMV-resistance mutation was M204I with L180M (48% of patients). M204V with L180M developed in 30% patients. The M204I and M204V mutations developed in 20% and 3% patients, respectively. Baseline HBV DNA levels did not differ between the four types of LMV-resistant mutations, as shown in Table 2.
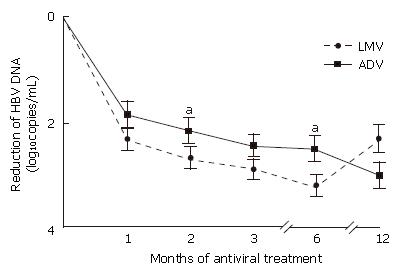
The decline in serum HBV DNA from the baseline level after LMV or ADV treatment was measured using real time PCR and the results are shown in Figure 1 as log10 copies/mL (mean ± SD). After one month of antiviral treatment, the mean reduction in HBV DNA levels was 2.3 and 1.8 during LMV and ADV treatment respectively (P = 0.121). However, after two months of treatment, LMV treatment produced a significantly greater decline in the serum HBV DNA level compared to ADV (2.7 ± 1.2 vs 2.1 ± 1.2; P = 0.021). Furthermore, after six months of treatment, HBV DNA levels fell by 3.2 ± 1.4 with LMV treatment compared to 2.5 ± 1.6 with ADV treatment (P = 0.030).
At treatment month 12, HBV DNA reduction from baseline in the LMV and ADV treatment groups was 2.3 and 3.0, respectively (P = 0.181). During each 12 mo of treatment with LMV and then ADV, 14 (32%) patients and two (5%) patients developed V-BT, respectively.
When ADV treatment was started, LMV therapy was continued for the first 1-3 mo in 17 patients. The reduction in HBV DNA from the baseline level during ADV treatment was not different between those receiving LMV/ADV combination therapy compared to those receiving ADV alone. HBV DNA reduction from baseline level was not different in the four types of LMV-resistant mutations.
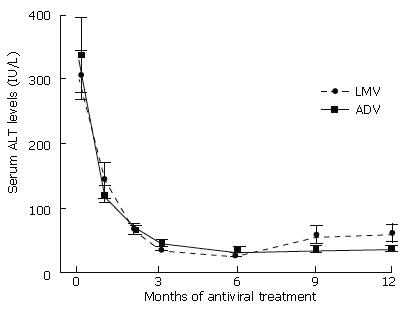
Figure 2 shows the changes in serum ALT levels before and during antiviral treatment. The mean serum ALT levels during 12 mo of antiviral treatment were not different between LMV and ADV treatment regimens. Serum ALT levels normalized in 40 (91%) patients during 12 mo of LMV treatment, and in 39 (86%) patients during 12 mo of ADV treatment (P = 0.551). The time taken to ALT normalization was 4.0 ± 3.55 mo and 5.3 ± 5.18 mo after the commencements of LMV and ADV treatment respectively (P = 0.081).
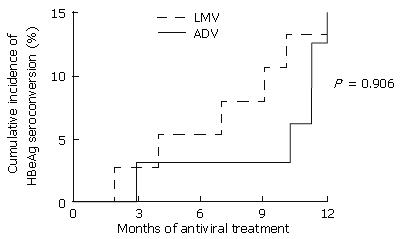
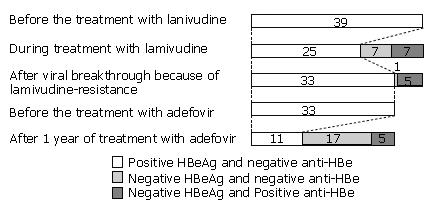
The cumulative rates of sustained HBeAg seroconversion during one year of antiviral treatment were not different between LMV and ADV (Figure 3). Cumulative rates after one year of treatment with LMV and ADV were 13% (5 of 39 patients) and 15% (5 of 33 patients), respectively. Two (40%) of the five patients whose HBeAg had seroconverted during one year of LMV treatment, reconverted to HBeAg positive status during V-BT. The changes in the HBeAg and anti-HBe status before and during treatment with LMV are shown in Figure 4.
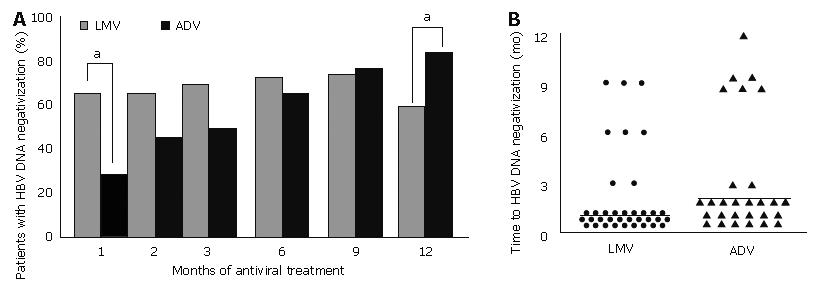
HBV DNA negative conversion (based on hybridization assay) was found in 27% patients after one month of ADV treatment, compared with 64% of patients after one month of LMV therapy (Figure 5A; P = 0.001). The proportion of patients who achieved HBV DNA negative conversion increased with the duration of ADV treatment to 83% after 12 mo of treatment. By contrast, HBV DNA negative conversion decreased to 59% after 12 mo of LMV treatment, because of the development of V-BT.
The mean time to HBV DNA negative conversion from the beginning of antiviral treatment was longer in the ADV treatment group (3.5 ± 2.9 mo; median 2 mo) compared to the LMV treatment group (2.0 ± 2.1 mo; median 1 mo) (Figure 5B; P = 0.020).
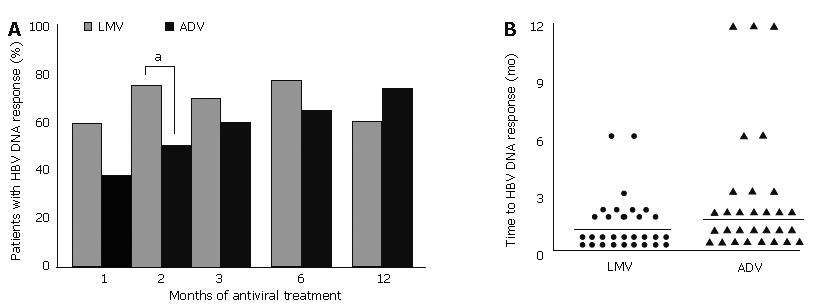
HBV DNA response was seen in 59% and 38% patients after one month of LMV and ADV treatment respectively (P = 0.46). After two months of treatment, HBV DNA response was seen in 51% patients on ADV compared to 74% on LMV treatment (Figure 6A; P = 0.026). The HBV DNA response rate increased to 73% after 12 mo of treatment with ADV. By contrast, HBV DNA response decreased to 61% after 12 mo of LMV treatment, because of the development of V-BT.
The mean time to HBV DNA response from the beginning of antiviral treatment was longer with ADV treatment (2.9 ± 3.4 mo; median 1.5 mo) than LMV treatment (1.6 ± 1.2 mo; median 1 mo; Figure 6B, P = 0.001).
The antiviral efficacy of ADV has been reported to be similar to that of LMV in nucleoside naïve patients with CHB[25,26,33,34]. Moreover, a recent in vitro study showed that LMV-resistant mutants remained sensitive to ADV[27]. These findings have been supported by several clinical studies, which showed similar antiviral efficacy of ADV against wild type HBV and LMV resistant HBV[28,29,35-37]. In these studies, HBV DNA reduction after ADV treatment in nucleoside-naïve patients and LMV-resistant patients were 2.9-3.9 and 2.5-4.3 log10 copies/mL, respectively. However, the antiviral efficacy of ADV in patients with LMV-resistance appears to be slower and less potent compared with the response to LMV in nucleoside-naïve patients; although the evidence in support of this observation is limited. The present study was designed to provide an answer to this question by direct comparison of antiviral efficacy of LMV and ADV using serum samples collected sequentially from patients with CHB who had earlier received LMV, and who had been switched to ADV because of the appearance of LMV resistance.
In our study, HBV DNA levels were quantified by two methods; hybridization assay and real time PCR. Real-time PCR has a very high sensitivity, although it has not been widely used clinically until recently. At the time our patients were under treatment with LMV, real time PCR was an experimental technique and most clinicians were using the hybridization assay to quantify HBV DNA. Therefore, the majority of studies have used such data to compare the antiviral efficacy of ADV in LMV-resistant patients with that of LMV in nucleoside-naïve patients. To assess the antiviral efficacy of LMV and ADV, it therefore seemed necessary to analyze the data obtained by the hybridization assay.
In our study, the suppression of serum HBV DNA levels after two and six months of treatment was lower with ADV compared to LMV. This finding is in contrast with a recent report that indicated that LMV-resistant mutations resulted in increased van der Waals contacts between ADV and the mutated residues, accounting for the superior binding affinity of ADV with these mutants[38]. Recently, Ono et al[39] reported that the median effective concentration values of ADV for LMV-resistant mutants were 4-16 times higher than those for wild-type HBV, and suggested that higher doses of ADV will be required for the treatment of LMV-resistant mutants. Our results support this suggestion.
To assess the antiviral efficacy of LMV and ADV, we compared the proportion of patients with HBV DNA negative conversion, and the time required to achieve this after LMV and ADV treatment. After one month of treatment, the conversion rate was significantly lower with ADV compared to LMV treatment (27% vs 64%). In addition, HBV DNA negative conversion took significantly longer after ADV than LMV. We also analyzed the proportion of patients with HBV DNA responses, and the time taken after LMV and ADV treatments. An HBV DNA response was defined as an HBV DNA level ≤ 105 copies/mL or a ≥ 2 log10 reduction from the baseline HBV DNA level, according to the criteria proposed by Perrillo et al[30]. In their study, HBV DNA response occurred in 85% of LMV-resistant patients after one year of treatment with ADV. Similarly, Locarnini et al[40] defined the antiviral response as ≥ 1 log10 reduction in HBV DNA from the baseline level within three months of treatment. In our study, HBV DNA response rate after two months of treatment was significantly lower with ADV compared to LMV (51% vs 74%). The HBV DNA response was also significantly delayed after ADV treatment compared with LMV.
In our study, 17 of the 44 patients treated with ADV also received LMV for the initial 1-4 mo of ADV treatment. Because such a combination could influence the overall antiviral efficacy, we compared the efficacy of HBV DNA suppression between patients who received combination treatment and those who did not, and found no significant difference in the results (data not shown).
Although the antiviral efficacy of LMV was faster than that with ADV during the first several months of treatment, the development of V-BT reduced this advantage of LMV after 12 mo of treatment. By contrast, the antiviral efficacy of ADV increased with time, with a low incidence of ADV-resistance. Therefore, ADV appears to be superior to LMV under conditions that require long-term antiviral treatment. When we compared the rate of HBV DNA reduction from the baseline level after the exclusion of patients who developed V-BT within 12 mo of antiviral treatment, there was no significant difference between LMV and ADV treatments.
The probability of a mutant strain being selected during therapy depends upon the ability of a drug to suppress viral replication[41]. Using a more potent antiviral drug during the initial course of treatment may reduce the chances of selection of drug resistant mutants[40,41]. Therefore, it is necessary to determine whether using ADV rather than LMV for the initial treatment might affect the incidence of drug resistance in long-term nucleoside/nucleotide-treated patients. In our study, only two of 44 (5%) patients developed V-BT after 12 mo of treatment because of ADV-resistant mutation, which was significantly lower than the incidence of V-BT caused by LMV-resistant mutations (32%, P = 0.002). However, the incidence of V-BT caused by ADV-resistant mutations was higher than that reported previously[24]. Our findings are consistent with a recent study which showed that the emergence of the ADV mutations in LMV-resistant patients appeared to occur earlier and was more frequent than in nucleoside-naïve patients[42]. Further studies are needed to determine whether the less potent and slower antiviral efficacy of ADV in the early treatment course in LMV-resistant patients could lead to a higher incidence of ADV-resistant mutations.
The main limitation of our study was that we compared the efficacy of ADV in LMV-resistant patients with that of LMV in nucleoside-naïve patients and not with that of ADV in nucleoside-naïve patients. It should be noted that we compared the antiviral efficacy of two different drugs, nucleoside analogue (LMV) and nucleotide analogue (ADV) under different conditions: a nucleoside-naïve state and an LMV-resistant state. However, our study was designed to analyze any difference in the antiviral efficacy, and the time taken to achieve sufficient HBV DNA suppression after commencement of antiviral therapy when LMV was switched to ADV because LMV resistance had developed. We demonstrated that ADV had slower and less potent antiviral effect, which most clinicians have suspected until now using the hybridization assays, and verified these findings using real time PCR.
In conclusion, the antiviral efficacy of ADV in patients with LMV-resistant HBV appears to be slower and less potent than that of LMV against wild type HBV during the early course of treatment. However, the superior initial antiviral efficacy of LMV was reduced in the later course of treatment because of the appearance of drug resistant viral mutations.
S- Editor Wang J L- Editor Anand BS E- Editor Liu Y
| 1. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1712] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 2. | Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 3. | Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis. 2002;2:395-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 610] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 4. | Conjeevaram HS, Lok AS. Management of chronic hepatitis B. J Hepatol. 2003;38 Suppl 1:S90-S103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Suzuki Y, Kumada H, Ikeda K, Chayama K, Arase Y, Saitoh S, Tsubota A, Kobayashi M, Koike M, Ogawa N. Histological changes in liver biopsies after one year of lamivudine treatment in patients with chronic hepatitis B infection. J Hepatol. 1999;30:743-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 574] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 7. | Jarvis B, Faulds D. Lamivudine. A review of its therapeutic potential in chronic hepatitis B. Drugs. 1999;58:101-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1381] [Cited by in RCA: 1347] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 9. | Tipples GA, Ma MM, Fischer KP, Bain VG, Kneteman NM, Tyrrell DL. Mutation in HBV RNA-dependent DNA polymerase confers resistance to lamivudine in vivo. Hepatology. 1996;24:714-717. [PubMed] |
| 10. | Ling R, Mutimer D, Ahmed M, Boxall EH, Elias E, Dusheiko GM, Harrison TJ. Selection of mutations in the hepatitis B virus polymerase during therapy of transplant recipients with lamivudine. Hepatology. 1996;24:711-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 350] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 11. | Leung NW, Lai CL, Chang TT, Guan R, Lee CM, Ng KY, Lim SG, Wu PC, Dent JC, Edmundson S. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology. 2001;33:1527-1532. |
| 12. | Liaw YF, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Chien RN, Dent J, Roman L, Edmundson S. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. Gastroenterology. 2000;119:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 519] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 13. | Chien RN, Liaw YF, Atkins M. Pretherapy alanine transaminase level as a determinant for hepatitis B e antigen seroconversion during lamivudine therapy in patients with chronic hepatitis B. Asian Hepatitis Lamivudine Trial Group. Hepatology. 1999;30:770-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 189] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Hadziyannis SJ, Vassilopoulos D. Hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2001;34:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 363] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 15. | Lai CL, Dienstag J, Schiff E, Leung NW, Atkins M, Hunt C, Brown N, Woessner M, Boehme R, Condreay L. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis. 2003;36:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 493] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 16. | Mutimer D, Pillay D, Shields P, Cane P, Ratcliffe D, Martin B, Buchan S, Boxall L, O'Donnell K, Shaw J. Outcome of lamivudine resistant hepatitis B virus infection in the liver transplant recipient. Gut. 2000;46:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Liaw YF, Chien RN, Yeh CT, Tsai SL, Chu CM. Acute exacerbation and hepatitis B virus clearance after emergence of YMDD motif mutation during lamivudine therapy. Hepatology. 1999;30:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 403] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 18. | Ben-Ari Z, Pappo O, Zemel R, Mor E, Tur-Kaspa R. Association of lamivudine resistance in recurrent hepatitis B after liver transplantation with advanced hepatic fibrosis. Transplantation. 1999;68:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Rizzetto M, Marzano A, Lagget M. Treatment of hepatitis B e antigen-negative chronic hepatitis B with lamivudine. J Hepatol. 2003;39 Suppl 1:S168-S171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Honkoop P, de Man RA, Niesters HG, Zondervan PE, Schalm SW. Acute exacerbation of chronic hepatitis B virus infection after withdrawal of lamivudine therapy. Hepatology. 2000;32:635-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 170] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Yuen MF, Kato T, Mizokami M, Chan AO, Yuen JC, Yuan HJ, Wong DK, Sum SM, Ng IO, Fan ST. Clinical outcome and virologic profiles of severe hepatitis B exacerbation due to YMDD mutations. J Hepatol. 2003;39:850-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Thabut D, Ratziu V, Bernard-Chabert B, Poynard T, Benhamou Y, Thibault V. Unsuccessful rescue therapy with adefovir dipivoxil for lamivudine resistant HBV in a patient with liver failure. Gut. 2003;52:614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Andreone P, Gramenzi A, Cursaro C, Biselli M, Cammà C, Trevisani F, Bernardi M. High risk of hepatocellular carcinoma in anti-HBe positive liver cirrhosis patients developing lamivudine resistance. J Viral Hepat. 2004;11:439-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Angus P, Vaughan R, Xiong S, Yang H, Delaney W, Gibbs C, Brosgart C, Colledge D, Edwards R, Ayres A. Resistance to adefovir dipivoxil therapy associated with the selection of a novel mutation in the HBV polymerase. Gastroenterology. 2003;125:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 422] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 25. | Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1060] [Cited by in RCA: 1019] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 26. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Wulfsohn MS. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 735] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 27. | Xiong X, Flores C, Yang H, Toole JJ, Gibbs CS. Mutations in hepatitis B DNA polymerase associated with resistance to lamivudine do not confer resistance to adefovir in vitro. Hepatology. 1998;28:1669-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 187] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | Perrillo R, Schiff E, Yoshida E, Statler A, Hirsch K, Wright T, Gutfreund K, Lamy P, Murray A. Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology. 2000;32:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 308] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 29. | Benhamou Y, Bochet M, Thibault V, Calvez V, Fievet MH, Vig P, Gibbs CS, Brosgart C, Fry J, Namini H. Safety and efficacy of adefovir dipivoxil in patients co-infected with HIV-1 and lamivudine-resistant hepatitis B virus: an open-label pilot study. Lancet. 2001;358:718-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 213] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 30. | Perrillo R, Hann HW, Mutimer D, Willems B, Leung N, Lee WM, Moorat A, Gardner S, Woessner M, Bourne E. Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology. 2004;126:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 305] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 31. | Ono Y, Onda H, Sasada R, Igarashi K, Sugino Y, Nishioka K. The complete nucleotide sequences of the cloned hepatitis B virus DNA; subtype adr and adw. Nucleic Acids Res. 1983;11:1747-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 337] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Hong SP, Kim NK, Hwang SG, Chung HJ, Kim S, Han JH, Kim HT, Rim KS, Kang MS, Yoo W. Detection of hepatitis B virus YMDD variants using mass spectrometric analysis of oligonucleotide fragments. J Hepatol. 2004;40:837-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Nowak MA, Bonhoeffer S, Hill AM, Boehme R, Thomas HC, McDade H. Viral dynamics in hepatitis B virus infection. Proc Natl Acad Sci USA. 1996;93:4398-4402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 576] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 34. | Tsiang M, Rooney JF, Toole JJ, Gibbs CS. Biphasic clearance kinetics of hepatitis B virus from patients during adefovir dipivoxil therapy. Hepatology. 1999;29:1863-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 180] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 35. | Werle B, Cinquin K, Marcellin P, Pol S, Maynard M, Trépo C, Zoulim F. Evolution of hepatitis B viral load and viral genome sequence during adefovir dipivoxil therapy. J Viral Hepat. 2004;11:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Peters MG, Hann Hw Hw, Martin P, Heathcote EJ, Buggisch P, Rubin R, Bourliere M, Kowdley K, Trepo C, Gray Df Df. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2004;126:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 423] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 37. | Schiff ER, Lai CL, Hadziyannis S, Neuhaus P, Terrault N, Colombo M, Tillmann HL, Samuel D, Zeuzem S, Lilly L. Adefovir dipivoxil therapy for lamivudine-resistant hepatitis B in pre- and post-liver transplantation patients. Hepatology. 2003;38:1419-1427. [PubMed] |
| 38. | Yadav V, Chu CK. Molecular mechanisms of adefovir sensitivity and resistance in HBV polymerase mutants: a molecular dynamics study. Bioorg Med Chem Lett. 2004;14:4313-4317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Ono SK, Kato N, Shiratori Y, Kato J, Goto T, Schinazi RF, Carrilho FJ, Omata M. The polymerase L528M mutation cooperates with nucleotide binding-site mutations, increasing hepatitis B virus replication and drug resistance. J Clin Invest. 2001;107:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 214] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 40. | Locarnini S, Hatzakis A, Heathcote J, Keeffe EB, Liang TJ, Mutimer D, Pawlotsky JM, Zoulim F. Management of antiviral resistance in patients with chronic hepatitis B. Antivir Ther. 2004;9:679-693. [PubMed] |
| 41. | Richman DD. The implications of drug resistance for strategies of combination antiviral chemotherapy. Antiviral Res. 1996;29:31-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Fung SK, Chae HB, Fontana RJ, Conjeevaram H, Marrero J, Oberhelman K, Hussain M, Lok AS. Virologic response and resistance to adefovir in patients with chronic hepatitis B. J Hepatol. 2006;44:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 253] [Article Influence: 13.3] [Reference Citation Analysis (0)] |