Published online Jan 21, 2007. doi: 10.3748/wjg.v13.i3.462
Revised: November 1, 2006
Accepted: November 27, 2006
Published online: January 21, 2007
AIM: To investigate the protective effect of stronger neo-minophafen C (SNMC) on fulminant hepatic failure (FHF) and its underlying mechanism.
METHODS: A mouse model of FHF was established by intraperitoneal injection of galactosamine (D-Gal N) and lipopolysaccharide (LPS). The survival rate, liver function, inflammatory factor and liver pathological change were obtained with and without SNMC treatment. Hepatocyte survival was estimated by observing the stained mitochondria structure with terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate fluorescence nick end labeling (TUNEL) method and antibodies against cytochrome C (Cyt-C) and caspase-3.
RESULTS: The levels of plasma tumor necrosis factor alpha (TNF-α), nitric oxide (NO), ET-1, interleukin-6 (IL-6), and the degree of hepatic tissue injury were decreased in the SNMC-treated groups compared with those in the model group (P < 0.01). However, there were no differences after different dosages administered at different time points. There was a significant difference in survival rates between the SNMC-treated groups and the model group (P < 0.01). The apoptosis index was 32.3% at 6 h after a low dose of SNMC, which was considerably decreased from 32.3% ± 4.7% vs 5% ± 2.83% (P < 0.05) to 5% on d 7. The expression of Cyt-C and caspase-3 decreased with the prolongation of therapeutic time. Typical hepatocyte apoptosis was obviously ameliorated under electron microscope with the prolongation of therapeutic time.
CONCLUSION: SNMC can effectively protect liver against FHF induced by LPS/D-Gal N. SNMC can prevent hepatocyte apoptosis by inhibiting inflammatory reaction and stabilizing mitochondria membrane to suppress the release of Cyt-C and sequent activation of caspase-3.
- Citation: Yang BS, Ma YJ, Wang Y, Chen LY, Bi MR, Yan BZ, Bai L, Zhou H, Wang FX. Protective effect and mechanism of stronger neo-minophagen C against fulminant hepatic failure. World J Gastroenterol 2007; 13(3): 462-466
- URL: https://www.wjgnet.com/1007-9327/full/v13/i3/462.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i3.462
Fulminant hepatic failure (FHF) is clinically characterized by prolonged prothrombin time and hepatic encephalopathy. It has been known that the onset of acute and chronic hepatitis is related to the inflammatory necrosis resulting from cell immunity and abnormal hepatocyte apoptosis caused by injury in mitochondria[1,2]. Stronger neo-minophagen C (SNMC), a compound mainly composed of glycyrrhizic acid, has anti-inflammatory and anti-allergic effects. However, it is not clear whether this compound protects liver against FHF[3]. The present study was to clarify the effect of SNMC on immune-mediated injury and hepatic cell apoptosis and its mechanism underlying liver failure induced by endotoxin.
Kunming mice weighing 18-20 g at the age of 6-7 wk were provided by the Center of Experimental Animals of Harbin Medical University. Female mice were never impregnated and reproduced. SNMC was obtained from Minofayan Pharmaceutical Co, Ltd, Japan. Galactosamine (D-Gal N) and lipopolysaccharide (LPS) were purchased from Sigma. Nitric oxide (NO) fluorescein reagent kit was obtained from Jian Cheng Bio-Engineering Research Institute (Nanjing, China). Tumor necrosis factor alpha (TNF-α), ET-1, interleukin-6 (IL-6) radioimmunoassay reagent kits were bought from Radioimmunoassay Institute, General Hospital of Chinese PLA. Caspase-3 monoclonal antibody was from Sigma. Anti cytochrome C (Cyt-C) antibody immunohistochemistry staining reagent kit and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate fluorescence nick end labeling (TUNEL) reagent kit were purchased from Zhongshan Biotechnology Co, Ltd (Beijing, China).
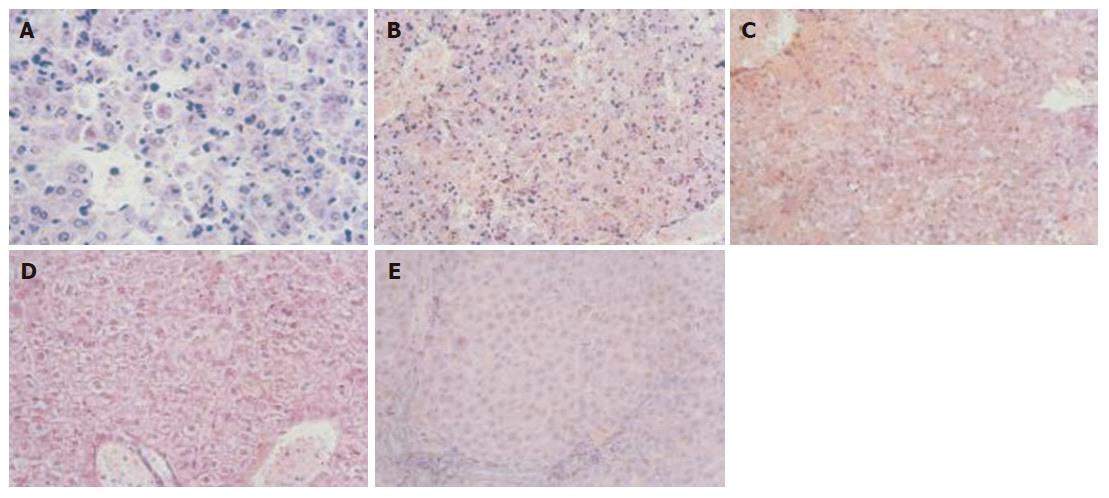
D-Gal N (1000 mg/kg) and LPS (100 μg/kg) were injected into the abdominal cavity of mice to induce FHF. Pathological changes in liver tissues corresponded to the FHF pathological features (Figure 1A and B). One hundred and ten mice were randomly divied into control group, model group and treatment group. Mice in the control group were administered SNMC for a week before LPS/D-Gal N treatment. Mice in the model group were given SNMC and LPS/D-Gal N at the same time. Mice in the treatment group were injected with LPS/D-Gal N 8 h after SNMC was given. The results showed that 280 mL, 200 mL and 140 mL SNMC had the most significant protective effect. The survival rate of mice in the model group after injected 140 mL SNMC was the highest. Then 60 mice were given 140 mL SNMC and 5 mice in each group were sacrificed 6 h, 1 d, 3 d and 7 d after LPS/D-Gal N injection. Liver tissue was collected for immunohistochemical analysis. Pyrogen free blood sample was obtained from eyeball and centrifuged for 10 min at 3000 r/min to get serum/plasma. Serum and plasma were stored at -20°C.
TNF-α, ET-1, IL-6 were determined by radioimmunoassay (RIA). The nitrate reductase method was employed to analyze NO. Alanine aminotransferase (ALT), total bilirubin (TBIL) and albumin (ALB) were analyzed with fully automatic biochemical instruments.
Four pieces of the left lobe of liver were taken. One was fixed in 2.5% glutaraldehyde for electron microscopy (EM), 2 were fixed in 4% paraformaldehyde and embedded in paraffin for immunohistochemical analysis, and 1 was fixed in 10% formalin and embedded in paraffin for histological examination.
Routinely deparaffinaged and rehydrated sections were stained with cell apoptosis detection kit according to the manufacturer’s instructions. The sections were visualized with diaminobenzidine (DAB). The apoptosis index was calculated and the degree of hepatocyte apoptosis was determined. The cells with brown nuclei and cytoplasm were regarded as apoptotic cells, whereas the cells with blue nuclei were considered normal cells. The sections were incubated with monoclonal antibodies against caspase-3 (1:100) and Cyt-C antibody (1:100) after routine deparaffinage and rehydration, and stained with DAB and counterstained with hematoxylin.
The data were expressed as mean ± SD. All statistical analyses were performed using the Statistical Program for Social Sciences (SPSS 10.0 for Windows). F test was used for comparing variables. The 95% confidence interval for all estimates was provided when appropriate. P < 0.05 was considered statistically.
All the mice of the control group were alive at the end of experiments. Death occurred in the model group at 6 h after LPS/D-Gal N treatment and all mice died within 21 h. Death occurred in the SNMC-treated group at 8 h and all the survivors lived for more than 24 h. The survival rate for the model group was 60% after 140 mL SNMC was given.
In the control group, the structure of hepatic lobules was intact, and the hepatocytes were radially arranged around the central vein. In the model group, histological hepatocyte damage with slight hydropic degeneration could be seen 3-5 h after the administration of D-Gal N and LPS. Furthermore, pronounced hydropic degeneration, slight spotty necrosis and apoptotic bodies (Figure 1A) could be observed 6 after administration of D-Gal N and LPS. Widespread necrosis (exceeding 2/3 of the whole slide), dissociated hepatocytes with loss of the normal structure of liver, hemorrhage and neutrophil and lymphocyte infiltration were seen mainly in necrosis area and periportal zone 12 h after administration of D-Gal N and LPS (Figure 1B). However, various degrees of hydropic and vacuolar degeneration were found in the model group 24 h after administration of 140 mL SNMC (Figure 1C). In the model group, marked cytoplasm rarefaction was found 36-48 h after administration of D-Gal N and LPS (Figure 1D), while sporadic and focal necrosis (Figure 1E) as well as inflammatory cell infiltration were found 72 h after administration of D-Gal N and LPS.
The F values were determined in the control, model and treatment groups. The results were as follows: F (NO) = 58.328, F (TNF-α) = 69.489, F (ET-1) = 57.328, F (IL-6) = 46.914, F (ALT) = 3.257, F (TBIL) = 7.477, [F(4,25)0.05 = 2.76, P < 0.05]. F (ALB) = 1.204, NS. The F values in the treatment group were as follows: F (NO) = 2.676, F (TNF-α) = 2.043, F (ET-1) = 3.347, F (IL-6) = 2.676, F (ALT) = 0.452, F (ALB) = 1.535, F (TBIL) = 0.020, [F(2,12)0.05 = 3.68, NS]. The F values in different groups injected with different dosages of SNMC were as follows: F (NO) = 84.907, F (TNF-α) = 44.519, F (ET-1) = 13.236, F (IL-6) = 37.760, F (ALT) = 2.904, F (TBIL) = 7.681, [F(4,25)0.05 = 2.76, P < 0.05), F (ALB) = 1.004, NS. The results of different dosages were: F (NO) = 3.351, F (TBIL) = 2.756, [F(2,12)0.05 = 3.68, NS], with no significant differences (Table 1).
| Groups | Mice (n) | NO (μmol/L) | TNF-α (ng/mL) | ET-1 (pg/mL) | IL-6 (pg/mL) | ALT (u/L) | ALB (g/L) | TBIL (u/L) |
| Control group | 6 | 45.1 ± 14.01 | 0.77 ± 0.08 | 50.8 ± 7.58 | 57.07 ± 12.67 | 67.17 ± 12.62 | 32.18 ± 4.16 | 9.2 ± 8.75 |
| Model group | 6 | 725.94 ± 156.94 | 3.75 ± 0.50 | 309.41 ± 38.45 | 413.56 ± 69.02 | 1406.33 ± 47.42 | 21.13 ± 3.66 | 70.03 ± 17.22 |
| Treatment group | 6 | 199.42 ± 85.98 | 1.34 ± 0.31 | 129.11 ± 17.97 | 155.22 ± 59.37 | 799.33 ± 171.19 | 20.03 ± 3.01 | 8.87 ± 0.85 |
| 140 mL group | 6 | 215.01 ± 28.72 | 1.35 ± 0.17 | 183.07 ± 75.93 | 149.81 ± 44.69 | 25.96 ± 6.35 | 26.10 ± 1.97 | 59 ± 29.31 |
| 200 mL group | 6 | 87.88 ± 12.46 | 1.34 ± 0.31 | 157.41 ± 35.64 | 133.89 ± 30.48 | 1169 ± 149.92 | 20.9 ± 1.28 | 27.33 ± 2.94 |
| 280 mL group | 6 | 184.54 ± 19.84 | 1.27 ± 0.27 | 216.15 ± 24.47 | 179.97 ± 65.09 | 24.33 ± 8.02 | 21.37 ± 5.44 | 51.5 ± 14.94 |
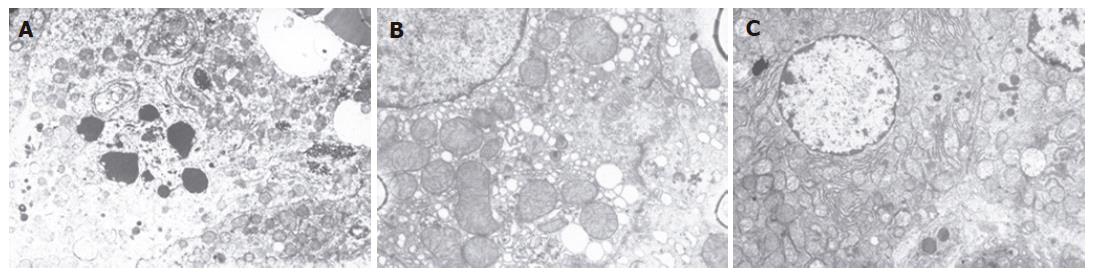
In the control group, the hepatocytes with prominent nuclei were well arranged in plates and abundant in structure of intracellular membrane. However, the model group showed condensed nuclei, chromatin margination, enlarged mitochondria, and derangement of plasmalemma and inner cytomembrane (Figure 2A). Condensed nuclei, chromatin margination, and enlarged rough endoplasmic reticulum (RER) showing degranulation were present in the 140 mL SNMC group. Normal bilayer nuclear membrane, abundant crista mitochondria, moderate density of matrix, tight cholangiole junctions and intact endothelium of the hepatic sinusoid were observed 3 d after administration of SNMC (Figure 2B). After 7 d, the structure of liver cell membranes was intact, the cell organs were rich, the crista mitochondria were dense, the matrix density was moderate, and the structure of hepatic sinusoid endothelia was intact. We could see the penetration of liver cell microvilli inside the Disse interspace (Figure 2C).
Two sections from each group were observed at each indicated time. Five visual fields of each section were analyzed, and 100 cell nuclei were counted in each visual field. The average percentage of hepatocyte apoptosis was considered the index of apoptosis[4] (Table 2). The apoptotic cells were characterized by intact bilayered cytomembrane and condensed nuclei. Nuclei and/or cytoplasm with brown staining (leakage of nuclear DNA) were assessed as positive staining. Besides, the apoptotic bodies were also positively stained. No apoptotic cells were found in the control group. More apoptotic liver cells were found mainly in the necrosis area of the model group with the prolongation of time.
| Groups | Number | Apoptosis index | Cyt-C | Caspase-3 |
| Control group | 5 | 0 | 1.67 ± 0.90 | 3.87 ± 2.42 |
| Model group | 5 | 33.2 ± 4.37 | 59.47 ± 3.79 | 85.6 ± 6.25 |
| After therapy 6 h | 5 | 32.3 ± 4.7 | 58.47 ± 3.83 | 84.00 ± 5.54 |
| After therapy 1 d | 5 | 26.6 ± 4.67b | 46.33 ± 5.51b | 72.3 ± 5.21b |
| After therapy 3 d | 5 | 19.9 ± 3.54b | 31.53 ± 2.83b | 56.13 ± 6.87b |
| After therapy 5 d | 5 | 11.1 ± 2.77b | 25.47 ± 3.94b | 34.1 ± 4.87b |
| After therapy 7 d | 5 | 5 ± 2.83b | 15.00 ± 4.11b | 17 ± 4.5b |
Cytoplasm with brown staining was assessed as positive. Three fields randomly selected from each section were chosen to detect Cyt-C and caspase-3 positive cells under optical microscope at a magnification of 400. The mean value was considered the positive cell number of each group (Table 2). In the control group, hepatocytes were normal in size and shape and well arranged. Cyt-C and caspase-3 were expressed only in a few scattered cells. In the model group, liver cells were mainly expressed in the inflammation necrosis area and portal area, and showed positive Cyt-C and caspase-3 staining. In the treatment group, the expression of Cyt-C and caspase-3 was gradually decreased and the structure of liver cells was gradually recovered with the prolongation of time.
It is well-known that virus infection results in primary liver injury and endotoxin, by which inflammatory mediator-induced secondary liver injury is caused, leading to hepatocyte apoptosis and necrosis[5]. TNF-α, one of the most important inflammatory mediators, induces hepatocyte apoptosis and necrosis through the activation of caspase-3, leading to liver cell DNA shift[6]. In the present study, SNMC reduced serum level of ALT, ALB and total bilirubin, and attenuated hepatocyte apoptosis, leading to the secondary liver injury induced by LPS. Its related mechanisms such as reducing the release of NO and ET-1, restraining the formation of hepatic sinusoid microthrombi and microcirculation dysfunction, inhibiting immunologic injury caused by cytokines especially TNF-α, and suppressing the formation of endotoxin, may contribute to the protective effect against FHF[7]. However, in the present study, SNMC failed to raise the albumin levels during FHF, probably due to the longer half life of albumin.
Most researchers believe that abnormal hepatocyte apoptosis contributes significantly to the occurrence of FHF[8]. The crucial role of mitochondria and Cyt-C in hepatocyte survival and death has caused more and more attention. Some mediators in the mitochondria are closely associated with cell apoptosis, including Cyt-C, apoptosis inducing factor (AIF) and reactive oxygen species (ROS). Under the stimulation of apoptosis signals, augmented mitochondrial membrane permeability initiates a series of key changes including release of Cyt-C, decrease in mitochondrial transmembrane potential, alteration of the oxidation-reduction system inside the cells and intervention with the Bc1 gene family, thus finally leading to hepatocyte apoptosis[9].
TUNEL staining results indicated that the apoptosis index in the treatment group decreased from 32.3% at 6 h to 5% on d 7 after LPS/D-Gal N injection (P < 0.05). Typical morphological changes during apoptosis, including nucleus shrinkage and chromatin margination etc, were observed under electron microscope (EM) in the model group. The treatment group improved proportionately with the prolongation of therapeutic time. The results suggest that SNMC can effectively attenuate hepatocyte apoptosis.
The release of Cyt-C is a key event in the apoptosis process[10]. Cyt-C has a duplex function as an initiator to activate cell apoptosis and participate in electron transfer. Cyt-C shifting to the cytoplasm could bring about a cascade of reactions of caspases, finally activate caspase-3 and result in hepatocyte apoptosis. Immunohistochemistry staining demonstrated that Cyt-C and caspase-3 expression was significantly reduced in treatment group with the prolongation of therapeutic time when compared with the model group (P < 0.01), suggesting that the expression of caspase-3 is closely related with Cyt-C and possibly regulated by releasing Cyt-C. Meanwhile, we speculated that SNMC inhibited the progression of hepatocyte apoptosis mainly by stabilizing the mitochondrial membrane and inhibiting the release of Cyt-C and subsequent caspase-3 activations.
The present study demonstrated that SNMC not only reduced serum aminotransferase and bilirubin, but also attenuated the hepatocyte apoptosis. SNMC reduced the necrotic area and increased the survival rate of mice by promoting hepatocyte regeneration and recovery of denatured cells, and protecting the undamaged cells. However, the dosages of SNMC in the treatment of FHF and the therapeutic time have not yet been firmly established. The low dose administered in this experiment corresponds to ordinary clinical dosages. Whether high dose would bring about toxic effects or other side effects awaits future study.
In summary, the results of the present study support SNMC treatment for FHF, but the precise mechanism should be further studied.
It is well-known that virus infection results in primary liver injury and endotoxin, by which inflammatory mediator-induced secondary liver injury is caused, thus finally leading to hepatocyte apoptosis and necrosis. Most researchers believe that abnormal hepatocyte apoptosis contributes significantly to the occurrence of FHF.
SNMC, a compound mainly composed of glycyrrhizic acid, has anti-inflammatory and anti-allergic effects. However, it is not clear whether this compound protects liver against FHF. The present study was to clarify the effects of SNMC on immune-mediated injury and hepatic cell apoptosis and its related mechanisms underlying liver failure induced by endotoxin.
SNMC can effectively protect liver against FHF induced by LPS/D-Gal N. SNMC prevents hepatocyte apoptosis by inhibiting inflammatory reaction and stabilizing mitochondria membrane to suppress the release of Cyt-C and sequent activation of caspase-3.
SNMC can not only reduce serum aminotransferase and bilirubin, but also the mortality of patients with FHF.
SNMC, a compound mainly composed of glycyrrhizic acid, has anti-inflammatory and anti-allergic effects.
S- Editor Liu Y L- Editor Wang XL E- Editor Bi L
| 1. | Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1429] [Cited by in RCA: 1429] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 2. | Ryo K, Kamogawa Y, Ikeda I, Yamauchi K, Yonehara S, Nagata S, Hayashi N. Significance of Fas antigen-mediated apoptosis in human fulminant hepatic failure. Am J Gastroenterol. 2000;95:2047-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Song FW, Li XJ. The research of the protective effect of SNMC on histopathology of Liver. Zhonghua Xiandai Yixue Zazhi. 2001;11:24-25. |
| 4. | Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S. Essential roles of the Fas ligand in the development of hepatitis. Nat Med. 1997;3:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 378] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 5. | Zhang YS, Tu ZG. Regulation of alpha 1-adrenoceptor on rat hepatocyte apoptosis induced by D-galactosamine and lipopolysaccharide. Acta Pharmacol Sin. 2000;21:627-632. [PubMed] |
| 6. | Hoofnagle JH, Carithers RL, Shapiro C, Ascher N. Fulminant hepatic failure: summary of a workshop. Hepatology. 1995;21:240-252. [PubMed] |
| 7. | Alison MR, Sarraf CE. Liver cell death: patterns and mechanisms. Gut. 1994;35:577-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Wang YM, Feng GH, Li Y. Relationship of Tumor Necrosis Factor-α and hepatocytes apoptosis in fulminant hepatic failure. Zhonghua Neike Zazhi. 2002;31:410-412. |
| 9. | Xiang XX, Wang GJ, Cai X. Research of the therapy to prevent hepatocytes apoptosis on Fulminant liver failure. Linchuang Ganzangbing Zazhi. 2001;6:64-65. |
| 10. | Finkel E. The mitochondrion: is it central to apoptosis? Science. 2001;292:624-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 149] [Article Influence: 6.2] [Reference Citation Analysis (0)] |