Published online Aug 7, 2007. doi: 10.3748/wjg.v13.i29.4011
Revised: April 15, 2007
Accepted: April 4, 2007
Published online: August 7, 2007
AIM: To investigate the apoptotic effect of photoexcited titanium dioxide (TiO2) nanoparticles in the presence of visible light on human hepatoma cell line (Bel 7402) and to study the underlying mechanism.
METHODS: Cerium-element-doped titanium dioxide nanoparticles were prepared by impregnation method. Bel 7402 human hepatoma cells were cultured in RPMI 1640 medium in a humidified incubator with 50 mL/L CO2 at 37°C. A 15 W fluorescent lamp with continuous wavelength light was used as light source in the photocatalytic test. Fluorescence morphology and agarose gel eletrophoresis pattern were performed to analyze apoptotic cells.
RESULTS: The Ce (IV)-doped TiO2 nanoparticles displayed their superiority. The adsorption edge shifted to the 400-450 nm region. With visible light illuminated for 10 min, 10 μg/cm3 Ce (IV)-doped TiO2 induced micronuclei and significant apoptosis in 4 and 24 h, respectively. Hochest 33 258 staining of the fixed cells revealed typical apoptotic structures (apoptotic bodies), agarose gel electrophoresis showed typical DNA ladder pattern in treated cells but not in untreated ones.
CONCLUSION: Ce (IV) doped TiO2 nanoparticles can induce apoptosis of Bel 7402 human hepatoma cells in the presence of visible light.
- Citation: Wang L, Mao J, Zhang GH, Tu MJ. Nano-cerium-element-doped titanium dioxide induces apoptosis of Bel 7402 human hepatoma cells in the presence of visible light. World J Gastroenterol 2007; 13(29): 4011-4014
- URL: https://www.wjgnet.com/1007-9327/full/v13/i29/4011.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i29.4011
Application of titanium dioxide (TiO2) photocatalysis has received increasing attention since the first report of microbiocidal effects by Matsunaga et al[1] in 1985. Recent studies have investigated application of TiO2 in life science, especially in the field of cancer treatment[2-4]. In the presence of ultraviolet (UV) light with a wavelength less than 400 nm, nano TiO2 can induce cancer cell apoptosis and be used as a photosensitizer in photodynamic therapy for endobronchial and esophageal cancers[5].
However, TiO2 displays its high photoactivity only when it is irradiated by ultraviolet light due to its wide band gap (3.2 eV for anatase). Doping with impurities has been widely used to modify the properties of TiO2 by introducing new states in its electronic structure[6,7]. Because of the unique 4f electron configuration, lanthanide metal ions are ideal dopants to modify the electronic structure of TiO2[8-10]. For example, cerium element doping could introduce new energy level into band gap of nano TiO2, making it possible for light with a wavelength over 400 nm (generally named visible light) enough to excite electron jumping to conduct band from valence band[10].
As hole and electron separate successfully on the surface of nanoparticles, they could react with absorbed water or other adsorbates to produce reactive oxygen specimens such as OH∙ and H2O2[6]. These reactive radicals can destroy cell membrane structure[11,12] and induce micronuclei (MN)[13], even cause DNA damage[12,14].
Apoptotic cells can be characterized by a number of morphologic, molecular, and biochemical features, including shrinkage of cells, blebbing of cells and nuclear membranes, compaction and condensation of chromatin toward the nuclear periphery, and fragmentation of DNA into oligonucleosomes[15]. Detection of more than one of these parameters is essential for the clear characterization of apoptotic cells.
In this communication, we reported the occurrence of oxidant-mediated DNA damage resulting in apoptosis and induction of micronuclei (MN) by Ce-doped TiO2 nanoparticle (CDT) in the presence of visible light in Bel 7402 human hepatoma cells.
CDT samples were prepared by impregnation technique[6]. Cerium sulphate [Ce(SO4)2] aqueous solution was slowly dropped into nano-anatase TiO2 (13 nm) gel by vigorous stirring, and water-bathed for 2 h at 40°C, then mixture was filtered, dried at 120°C for 2 h and finally calcined at 500°C for 3 h. CDT was suspended in physiologic saline, sterilized and diluted with RPMI 1640 medium to appropriate concentration when exposed to cells.
The characteristics of CDT were determined with UV-Vis spectrometer (SPECORD 2000, German) by X-ray diffraction (XRD, DX-2000), and transmission electron microscopy (TEM, JEOL JEM-2011).
The present study was carried out using Bel 7402 human hepatoma cell line, which was cultured with RPMI 1640 (Gibco) containing 10% (v/v) fetal calf serum (FCS), 50 U/mL penicillin, 50 μg/mL streptomycin, and 1% (v/v) 0.2 mol/L L-glutamine. Cells were maintained at 37°C in a humidified atmosphere containing 50 mL/L CO2.
After attached to culture plate, Bel 7402 cells were exposed to CDT for 4 h in incubator, then washed three times softly with PBS and irradiated for 10 min with the 15 W fluorescent lamp at a vertical height of 30 cm.
Variations in cell morphology were analyzed by fluorescence microscopy. Cells attached to coverslips were fixed in cold methanol for 5 min, air dried, stained with Hoechest-33 258 (2.5 mg/mL in milli-Q water, 2 min). Microscopic observations were carried out under Olympus BX61 microscope, and photographs were taken with Olympus DP50 digital camera.
Fragmentation of DNA was analyzed by agarose gel electrophoresis during apoptosis. Bel 7402 cells were treated with 10 μg/cm3 CDT for 4 h, then irradiated for 10 min with fluorescent light, and incubated for 4 h or overnight.
DNA was extracted with phenol/chloroform and precipitated in ethanol. The extracted DNA was separated by agarose gel electrophoresis (1.5% agarose gel containing 0.4 μg/mL ethidium bromide, 100 V, 1 h) and visualized under UV light.
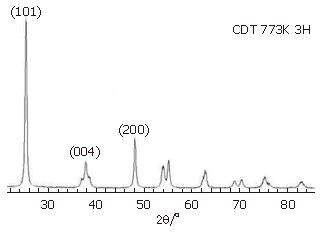
Figure 1 shows XRD patterns of CDT. The presence of peaks (2θ = 25.4, 37.8 and 48.1) could be regarded as an attributive indicator of anatase TiO2[10]. CDT could maintain anatase crystalline structure at 500°C, and no other crystal phases were observed. According to Scherrer formula, d = λ/β cosθ, particle size was about 21 nm.
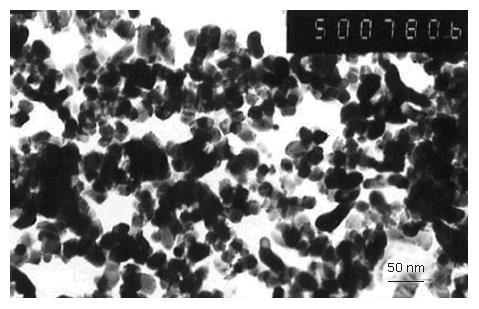
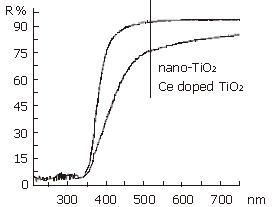
Morphologies of CDT were studied under transmission electron microscope (Figure 2). Most particles were in sphere shape, with a size of about 20-30 nm, coincided with that of XRD. Diffuse reflectance of UV-vis adsorption spectra of undoped TiO2 and CDT calcinated at 500°C was observed (Figure 3). The adsorption edge of CDT shifted to a longer wavelength region (named red shift) than that of undopd TiO2.
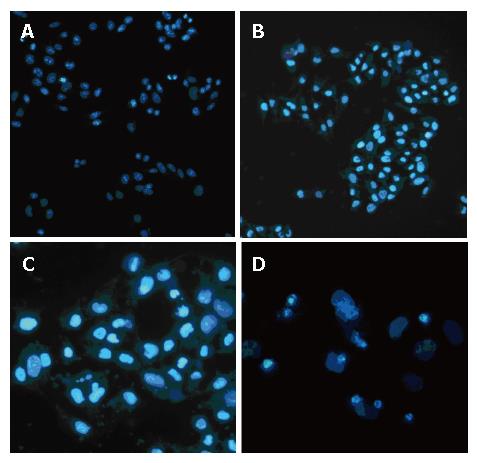
Bel 7402 cells were stained with Hoechest 33 258 (Figure 4). Neither CDT had effect on cells in the absence of light (Figure 4A), nor light (Figure 4B). Formation of MN could be found 4 h after cells were exposed to CDT (10 μg/cm3, with visible light illuminated for 10 min) (Figure 4C). Treated cells appeared typical changes in apoptosis after incubated overnight (Figure 4D). Many apoptotic bodies containing nuclear fragments were found in CDT-treated cells. Normal cells were stained uniformly, apoptotic cells appeared a brighter fluorescence. At the same time, cytoplasmic shrinkage was also observed.
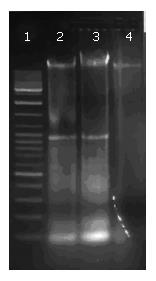
Fragmentation of CDT-treated DNA (at the concentration of 5 and 10 μg/cm3, irradiated for 10 min, incubated overnight) was found after agarose gel electrophoresis (Figure 5). In contrast, DNA in untreated cells did not show such a DNA ladder pattern in electrophoresis. There was no conspicuous difference in the effect of both concentrations.
In this experiment, CDTs were easy to attach to cellular membranes and aggregated. They were also easy to enter cytoplasm via phagocytosis (data not shown).
Nano TiO2 showed no cytotoxicity to cells in the absence of UV light[16], neither did UV light alone[17]. Visible light could only elevate culture temperature to 36°C, since it was lower than the culture temperature of cells, thermal death did not occur[18].
However, cell viability decreased as the concentration of UV-irradiated TiO2 nanoparticles in cells increased. Furthermore, cell morphology changed with increasing nanoparticle concentration, giving rise to shrinkage and fragmentation of cells[4].
DNA was damaged when exposed to UV and nano TiO2. Strand breaks in DNA were assayed on agarose gels by transformation of a supercoiled plasmid into the relaxed or linear form[14]. Nakagawa et al[12] studied the effects of four sizes of UV-irradiated TiO2 particles on a mouse lymphoma cell line. DNA tail length was measured by SCG assay. The results showed that UV-irradiated samples could increase DNA damage and decrease cell survival.
These observations have led the researchers to believe that the mechanism of cell death caused by photoexcited TiO2 nanoparticles is through reactive oxygen species (ROS).
Uchino et al[17] studied the relationship between the amount of radicals produced in UV irradiated TiO2 particles and cytotoxicity. The viability of Chinese hamster ovary (CHO) cells with internalized TiO2 particles decreased significantly after UV irradiation. Although the intensity of UV light did not influence cytotoxicity, the anatase fraction in TiO2 particles had a significant effect on cytotoxicity. In addition, cell viability was proportional to the formation of DMPO-OH radical adducts. The Electron spin resonance (ESR) results confirmed the presence of DMPO-OH radical adducts, consistent with the formation of OH∙ radicals. The optimum crystal size for OH∙ radical formation was 30 nm for anatase. Most anatase samples produced more hydroxyl radicals than rutile oram orphous TiO2. These findings indicate that when anatase forms TiO2 with UV exposure, hydroxyl radicals have cytotoxic effects[17].
Molecular scavengers of both hydrogen peroxide and hydroxyl radicals, catalase and cysteine could effectively diminish cell death when added to cell samples. These findings provide evidence that hydroxyl radicals and hydrogen peroxide play a role in cell death[14,17,19].
Cerium element doping can reduce band gap energy by introducing new energy into the surface band gap of nano TiO2[10]. The adsorption edge of nano TiO2 could shift to the long-wavelength region, and the energy of light at a length of 400-450 nm is enough to excite electron jump from valence band to conduct band by Ce (IV) doping. At the same time, Ce (IV) can separate e-/h+ pair by grasped electron, then liberate h+ to reacting H2O to generate ·OH and H2O2, leading to accumulation of ROS on cell membranes and in cytoplasm.
MN is frequently used to detect genetic damage induced by environmental mutagens, andror carcinogens. In general, MN may arise from the breakage of chromosomes or the loss of an entire chromosome[13]. Our results show that CDT at a noncytotoxic dose can significantly increase the frequency of MN after UV irradiation. The formation of MN may be due to ROS and/or the presence of these particles around the mitotic apparatus.
Apoptosis is an unique type of programmed cell death, and oxidant-mediated DNA damage is one of the key factors for its induction[20].
In the present study, Bel 7402 cells treated with photoexcited CDT were severely damaged, with cells contracted and a lot of cell fragments simultaneously observed under inverted phase-contrast microscope.
Degradation of internucleosomal DNA segments as a consequence of activation of endogenous endonucleases is considered a characteristic end point of apoptosis and can be analyzed by gel electrophoresis[21]. In the present study, DNA agarose gel electrophoresis depicted typical inter-nucleosomal fragmentation in DNA of CDT-treated cells.
Cell damage induced by nanoparticles occurs in two stages. The first stage is oxidative damage by the photoexcited TiO2 nanoparticles as they come in contact with cell membranes, resulting in permeabilization of the cell membranes but not producing a significant decrease in cell viability. The decrease in cell viability, eventually cell death, occurs as a result of leaking intracellular components, or CDT trafficking into the damaged cells and directly attacking nuclei and other intracellular components.
In conclusion, nano CDT induces apoptosis in the presence of visible light. However, the precise mechanism of MN formation and induction of apoptosis by ultra fine particles and fibers is still unknown. Further insight is required into intracellular signaling and the role of reactive oxygen species.
S- Editor Zhu LH L- Editor Wang XL E- Editor Li JL
| 1. | Matsunaga T, Tomoda R, Nakajima T, Wake H. Photoelectro-chemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol Lett. 1985;29:211-214. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1069] [Cited by in RCA: 1079] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 2. | Fujishima A, Cai RX, Otsuki J, Hashimoto K, Itoh K, Yamashita T, Kubota Y. Biochemical application of photoelectrochemistry: photokilling of malignant cells with TiO2 powder. Electrochim Acta. 1993;38:153-157. [RCA] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Fujishima A, Rao TN, Tryk DA. Titanium dioxide photo-catalysis. J Photochem Photobiol C. 2000;1:1-21. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6275] [Cited by in RCA: 3007] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 4. | Zhang AP, Sun YP. Photocatalytic killing effect of TiO2 nanoparticles on Ls-174-t human colon carcinoma cells. World J Gastroenterol. 2004;10:3191-3193. [PubMed] |
| 5. | Ackroyd R, Kelty C, Brown N, Reed M. The history of photodetection and photodynamic therapy. Photochem Photobiol. 2001;74:656-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 6. | Xie YB, Yuan CW. Visible-light responsive cerium ion modified titania sol and nanocrystallites for X-3B dye photodegradation. Appl Cataly B: Environ. 2003;46:251–259. [DOI] [Full Text] |
| 7. | Ulrike Diebold. The surface science of titanium dioxide. Surf Sci Rep. 2003;48:53. [DOI] [Full Text] |
| 8. | Anpo M, Takeuchi M. The design and development of highly reactive titanium oxide photocatalysts operating under visible light irradiation. J Catal. 2003;216:505. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1383] [Cited by in RCA: 670] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 9. | Xu AW, Gao Y, Liu HQ. The preparation, characterization, and their photocatalytic activities of rare-earth-doped TiO2 nanoparticles. J Catal. 2002;207:151. [RCA] [DOI] [Full Text] [Cited by in Crossref: 958] [Cited by in RCA: 963] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 10. | Hou TH, Mao J, Zhu XD, Tu MJ. STM and STS investigations of Ce-doped TiO2 nanoparticles. Rare Metals. 2006;25:331-336. [DOI] [Full Text] |
| 11. | Sakai H, Ito E, Cai RX, Yoshioka T, Kubota Y, Hashimoto K, Fujishima A. Intracellular Ca2+ concentration change of T24 cell under irradiation in the presence of TiO2 ultrafine particles. Biochim Biophys Acta. 1994;1201:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Nakagawa Y, Wakuri S, Sakamoto K, Tanaka N. The photogenotoxicity of titanium dioxide particles. Mutat Res. 1997;394:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 136] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Lu PJ, Ho IC, Lee TC. Induction of sister chromatid exchanges and micronuclei by titanium dioxide in Chinese hamster ovary-K1 cells. Mutat Res. 1998;414:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Dunford R, Salinaro A, Cai L, Serpone N, Horikoshi S, Hidaka H, Knowland J. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett. 1997;418:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 289] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Darzynkiewicz Z, Bruno S, Del Bino G, Gorczyca W, Hotz MA, Lassota P, Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1368] [Cited by in RCA: 1406] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 16. | Kubota Y, Shuin T, Kawasaki C, Hosaka M, Kitamura H, Cai R, Sakai H, Hashimoto K, Fujishima A. Photokilling of T-24 human bladder cancer cells with titanium dioxide. Br J Cancer. 1994;70:1107-1111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 123] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Uchino T, Tokunaga H, Ando M, Utsumi H. Quantitative determination of OH radical generation and its cytotoxicity induced by TiO(2)-UVA treatment. Toxicol In Vitro. 2002;16:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Cai RX, Hashimoto K, Itoh K, Kubota Y, Fujishima A. Photokilling of malignant cells with ultrafine TiO2 powder. Bull Chem Soc Jpn. 1991;64:1268–1273. [RCA] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 201] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Cai R, Kubota Y, Shuin T, Sakai H, Hashimoto K, Fujishima A. Induction of cytotoxicity by photoexcited TiO2 particles. Cancer Res. 1992;52:2346-2348. [PubMed] |
| 20. | Schothorst AA, Suurmond D, Schouten R. Photochemical damage to DNA treated with chlorpromazine and near UV radiation under aerobic and anaerobic conditions. Photochem Photobiol. 1983;38:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Rahman Q, Lohani M, Dopp E, Pemsel H, Jonas L, Weiss DG, Schiffmann D. Evidence that ultrafine titanium dioxide induces micronuclei and apoptosis in Syrian hamster embryo fibroblasts. Environ Health Perspect. 2002;110:797-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 249] [Article Influence: 10.8] [Reference Citation Analysis (0)] |