Published online Aug 7, 2007. doi: 10.3748/wjg.v13.i29.4006
Revised: March 3, 2007
Accepted: April 7, 2007
Published online: August 7, 2007
AIM: To explore the therapeutic efficacy and mechanism of herpes simplex virus-thymidine kinase (HSV-tk) targeting angiogenesis against hepatocellular carcinoma in vivio and in vitro.
METHODS: Recombinant adenovirus containing kinase domain insert with receptor (KDR) or cytomegalovirus (CMV) promoter-controlled HSV-tk gene (AdKDR-tk and AdCMV-tk) was constructed using pAdeasy system. The expression of KDR antigen in human umbilical venous endothelial cells (HUVEC) and HepG2 was detected with histological analysis of cells. The virus was used to infect HUVEC and HepG2. Following administration of ganciclovir (GCV), the survival rate of gene-transfected HUVEC and HepG2 was evaluated by MTT method. To develop hepatocarcinomas in 32 Balb/C mice with HepG2 cells, the mice were divided into four groups: ganciclovir group (I), Ad group (II), AdCMV-tk group (III) and AdKDR-tk group (IV). Then selective administration of recombinant adenovirus or Ad via the intratumorial was given to all rats. Ganciclovir (GCV) was given at a dose of 100 mg·kg-1·d-1 (ip) started on the following day and lasted 10 d. Microvessel density (MVD) of tumor in all the treated animals were examined by the immunohistochemical methods and tumor burden was evaluated 10 d before and after the last GCV dose.
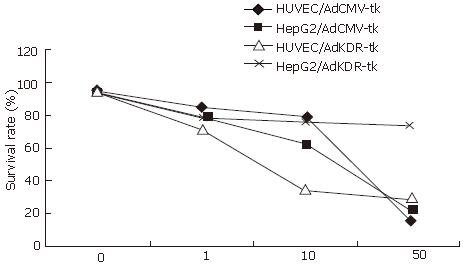
RESULTS: Immunocytochemical staining indicated the expression of KDR antigen in HUVEC. Under adenovirus infection index of 100, with increasing GCV concentration from 0 up to 50 mg/L, the survival rate of AdKDR-tk-transfected HUVEC and HepG2 decreased from 100% to (28.94 ± 5.67)% and (75.45 ± 2.91)% at proper order, respectively (P < 0.01), while the survival rate of AdCMV-tk-transfected HUVEC and HepG2 declined from 100% to (17.56 ± 2.48)% and (23.15 ± 5.72)%, respectively (P > 0.05). Compared with groupI, there was a decrease of tumor weight by 14.7% in group III and by 23.6% in group IV. And there was a distinct difference between group III and IV (P < 0.05). The median MVD for all groups was 37.4 ± 8.6, 30.6 ± 7.8, 27.6 ± 7.1, and 10.7 ± 4.1 (microvessels/mm2) in groupI, II, III and IV, respectively. And there was a marked difference between group III and II (P < 0.05), IV and II (P < 0.01), and IV and III (P < 0.01).
CONCLUSION: KDR promoter-HSV-tk gene may effectually restrain the growth of tumor via targeting angiogenesis for hepatocellular carcinoma with treatment of GCV.
- Citation: Li BJ, Zhang C, Yi YX, Hao Y, Liu XP, Ou QJ. Vascular damage and anti-angiogenic effects of tumor vessel-targeted adenovirus-mediated herpes simplex virus thymidine kinase gene. World J Gastroenterol 2007; 13(29): 4006-4010
- URL: https://www.wjgnet.com/1007-9327/full/v13/i29/4006.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i29.4006
Hepatocellular carcinoma (HCC) is the third leading cause of cancer death, with over a million new cases annually[1]. Because the success of the conventional therapies is limited, Gene and immunotherapy using a variety of strategies are in development at present. HCC possesses several characteristics that make it an attractive target for these therapies[2,3].
Solid tumors including HCC recruit new blood vessels to support tumor growth, and unique epitopes expressed on tumor endothelial cells can function as targets for the anti-angiogenic therapy of cancer[4-7]. KDR (kinase domain insert containing receptor, KDR) is one of two receptors (named KDR and flt-1 respectively) for vascular endothelial growth factor, a potent angiogenic peptide, and its expression is restricted to endothelial cells in vivo. Furthermore, it is expressed at a high level in the neoplasm vascular endothelial cells, but a low level in the normal ones[8]. Gene transfer into specific tissues or cell types is a key technique in the development of gene therapy. A tissue-specific promoter has been found to be potentially valuable for gene therapy, as it linked any other gene to be expressed specifically in target cells[9]. So, the KDR promoter is considered to be endothelial cell-specific, thus enabling targeted gene delivery to endothelial cells[10-12].
A suicide gene is a gene encoding an enzyme that converts nontoxic prodrugs into toxic forms[13]. The herpes simplex virus-thymidine kinase (HSV-tk) gene is one of the most widely studied suicide genes, as it can effectively damage the neoplasm vascular endothelial cells[14]. But the killing effect of currently used HSV-tk system is non-specific due to employment of cytomegalovirus (CMV) as its promoter.
In this study, we adopted anti-angiogenic strategy for the treatment of HCC. We evaluated the hepatoma-specific enhancement of GCV-mediated cytotoxicity induced by the HSV-tk gene under the control of KDR promoter in vivo.
Culture medium DMEM, OptiMEM and fetal bovine serum were purchased from Gibco, USA. GCV was purchased from Roche, USA. Adenoviral shuttle vector pAdTrack-CMV and pAdtrack (either of titer of the virus liquid was 1 × 1010 pfu/mL), the human umbilical venous endothelial cells (HUVEC) and the heaptoblastoma cell line HepG2 were kept in our lab.
Monolayer cultures of HUVEC and HepG2 cells were grown in DMEM medium supplemented with 10% fetal bovine serum at 37°C in 5% CO2.
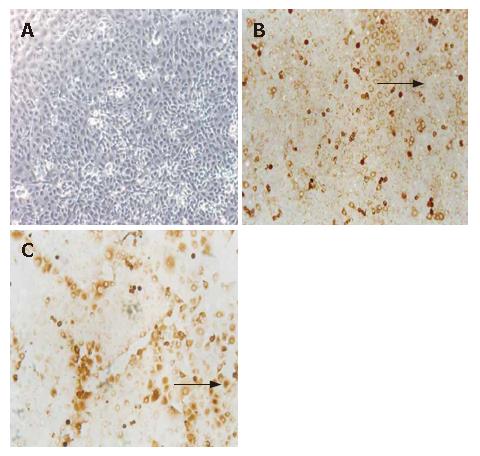
The immunocytochemical staining has been described previously[15]. Briefly, two slides (5 × 105 HepG2 or HUVEC cells/slide) were incubated with the monoclonal antibodies KDR (Sanbio, Uden, the Netherlands), and the same number of slides was incubated with isotype-specific irrelevant control monoclonal antibodies. The visualization step included the alkaline phosphatase/antialkaline phosphatase technique, and the slides were counterstained with hematoxylin to visualize nuclear morphology. HepG2 or HUVEC samples were also analyzed following the same procedures.
HUVEC or HepG2 cells were cultured in 96-well plates 2 × 103/well, and infected at a MOI (Multiplicity Of Infection) of 0, 1, 10, 100 and 1000 with AdCMV-TK or AdKDR-TK separately next day. After 16 h of infection, different concentrations of GCV (0, 1, 10 and 50 mg/L) were respectively added into the medium, and incubated for 5 d, but no GCV was added into the medium in the control group. MTT was added to each well to a final concentration of 200 mg/L. DMSO 150 μL was added 4 h later. Optical densities (OD) were measured at the wavelength of 490 nm. Each group set three repeated wells and each experiment was performed for three times.
Four-week-old male BALB/c-nu/nu athymic mice were purchased from Southern China Medical University Animal Centre (Guangzhan, China). The mice were maintained under a constant room temperature (25°C) and were provided with free access to a standard diet and tap water according to institutional guidelines. The study was approved by the Ethical Committee of Peking University Shenzhen Hospital (Shenzhen, China). The mice were inoculated s.c. in the right back with 1 × 107 cells of the human hepatocellular carcinoma HepG2 cell lines. Two wk later, when transplanted tumors had grown to 1 mm in diameter, 32 nude mice were subsequently divided into 4 groups (n = 8 each group): Ganciclovir group (I), Ad group(II), AdCMV-tk/GCV group (under the driving of CMV promoter) (III) and AdKDR-tk/GCV group (IV).Then the selective intratumoral injection of recombinant adenovirus or Ad was administered to all nude mice, and repeated 24 h later. The next day, the mice were given i.p. injections of GCV (100 mg/kg) daily for 10 d. The lethality of the method was 0%. Mice were monitored at least twice a week for evidence of tumor development, quantification of tumor size, and evidence of tumor-associated morbidity. Serial changes in tumor weight were estimated twice a week after the start of the GCV treatment.
Paraffin-embedded tissue sections (5 μm) were examined after stained with Mayer’s H&E (Sigma Chemical Co., St. Louis, MO). Monoclonal antibody against the endothelial cell marker, rat anti-mouse CD31 antibody (eBioscience, USA) was used. Then the primary antibody was detected by biotinylated secondary rabbit anti-rat IgG and SABC immunohistochemistry kit (Wuhan Boster Biotech, China) according to instructions of the manufacturer. Paraffin sections were also stained with HE to reveal tumor infiltrating lymphocytes and apoptosis of tumors.
Tumor microvessel density (MVD) was counted as previously described[16,17]. The CD31 staining sections were scanned under 40 × microscope and “hot spots” (area with greatest vascula) were found, from which 3 random fields of 200 × microscope were selected. The average vascular number of the three fields appeared as MVD. For HE staining section, 5 random non-necrotic tissue fields under 400 × microscope were selected to investigate the change of the tumor.
The statistical significance of differential findings between experimental groups and controls was determined by Student’s t test and considered significant if two-tailed P was < 0.05.
Two slides were immunostained with both KDR and control antibody. More positive cells were detected in HUVEC, without similar cells in the negative control, and no positive cells were detected in HepG2 (Figure 1).
HepG2 and HUVEC, infected with recombinant adenovirus pAdKDR-tk at various MOI, had different sensitivity to the GCV, while MOI = 100 was the most sensitive. At this time, density of GCV increased from 0 to 50 mg/L, and the survival rates of infected HUVEC and the HepG2 was descended to (28.94 ± 5.67) and (75.45 ± 2.91)% from 100%, respectively. Both had the obvious difference (Figure 2, P < 0.01). HepG2 and HUVEC, infected with different MOI of pAdCMV-tk, had similar sensitivity to the GCV. As the MOI and the GCV density increased, the cell survival rate all presented an obviously descending trend. When MOI was 100, concentration of GCV increased from 0 to 50 mg/L, the HUVEC and the HepG2 cell survival rates changed into (17.56 ± 2.48)% and (23.15 ± 5.72)%, respectively (Figure 2, P > 0.05). Under infection index of 100, with increasing GCV concentration from 0-50 mg/L, the survival rate of AdKDR-tk-transfected HUVEC and HepG2 decreased from 100% to (28.94 ± 5.67)% and (75.45 ± 2.91)% at proper order, respectively (P < 0.01), while the survival rate of AdCMV-tk-transfected HUVEC and HepG2 declined from 100% to (17.56 ± 2.48)% and (23.15 ± 5.72)%, respectively (Figure 2, P > 0.05).
On the next day after inoculation, the mice received gene therapy. No skin lesions were found in the injection sites. On d 21 after inoculation, tumors were dissected and weighed. Big tumors could be found in all mice of Ganciclovir group (I) and Ad group (II). In AdCMV-tk/GCV group (under the driving of CMV promoter) (III) and AdKDR-tk/GCV group (IV), all mice formed tumors, some of which were much smaller. The average tumor weight of AdKDR-tk/GCV group (IV) was much lower than that of AdCMV-tk/GCV group or Ad group (II) (P < 0.01). Compared with groupIthe tumor inhibitory rate was 12.3% (inhibitory efficiency = 1-tumor weight of adenovirus group/tumor weight of Ganciclovir group) in group III and 24.5% in group IV; and the inhibition rates were significantly different between groups III and IV (P < 0.05).
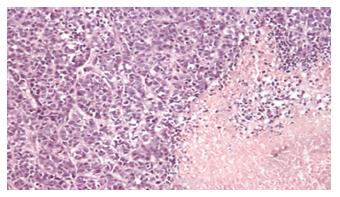
Tumor sections were stained with HE. There were abundance nucleus divisions with interstitial vessels, and no necrosis was found. There was necrosis in various degrees among groups II, III and IV. The necrosis in group IV was the most severe, and that in group II was the mildest (Figure 3).
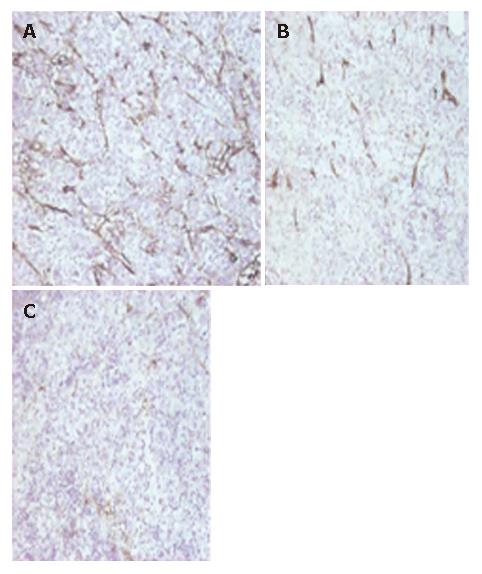
MVD was counted after CD31 staining in tumor sections. The mean MVD in groupsI, II, III and IV was 37.4 ± 8.6, 30.6 ± 7.8, 27.6 ± 7.1, and 10.7 ± 4.1 (microvessels/mm2), respectively. There were significant differences in the mean MVD between group III and II (P < 0.05), IV and II (P < 0.01), and IV and III (P < 0.01), but no significant differences between groupIand II (P > 0.05). Compared with Ganciclovir group (I) and Ad group (II), MVD of hepatoma treated with AdCMV-tk/GCV or AdKDR-tk/GCV was much lower. And in AdKDR-tk/GCV group, MVD was less than any other groups (P < 0.01), (Figure 4).
The realization that tumor growth requires intense neovascularization is the basis for a series of approaches aimed to specifically block the cancer-induced formation of new vessels. Anti-angiogenic therapy is supposed to be safe because it does not affect the mature vessels of normal tissues. Since HCC is known to be vascularized, antiangiogenic therapies may have a strong therapeutic benefit. Gene therapy may play an important role in this field, because anti-angiogenic factors need to be delivered for a long period to control the progression of tumors. In fact, an adenoviral vector carrying the endostatin cDNA was more effective than the direct injection of the protein[18].
Vascular endothelial growth factor (VEGF) plays a critical role in angiogenesis of HCC among important angiogenic factors involved in the regulation of angiogenesis in HCC[19].
KDR/flk-1 is the receptor for vascular endothelial growth factor, a potent angiogenic peptide[20,21], and its expression is restricted to endothelial cells in vivo. In addition, only KDR has been shown to autophosphorylate in the presence of VEGF[22]. Thus, the presence of KDR is absolutely required for the development of endothelial cells from hemangioblastic precursors. In 1995, Patterson C et al[11] first cloned and characterized the promoter of the human KDR gene. They found that it directed high level promoter activity in endothelial cells but not in other cell types. So, the KDR promoter is considered to be endothelial cell-specific, thus enabling targeted gene delivery to endothelial cells[23]. Jaggar RT[24] designed promoter sequences from the endothelial-specific KDR/VEGF receptor, and found that KDR has been shown to be upregulated on tumor endothelium. The herpes simplex virus thymidine kinase gene therapy with ganciclovir forms the basis of a widely used strategy for suicide gene therapy[25]. Mavria G[26] demonstrated that vascular targeting combined with HSV-TK is more effective. Recombinant adenoviruses provide a versatile system for gene expression studies and therapeutic applications. Adenoviruses transfer genes to a broad spectrum of cell types independent of active cell division. Additionally, high titers of viruses and high levels of transgene expression can be obtained[27].
Our study demonstrated that adenovirus-mediated transfection of KDR promoter-HSV-TK gene could yield a specific killing effect on vascular endothelial cells, and highlight the way to overcome the heterogeneity of tumors. And pronounced tumor regressions can be achieved in mice with adenovirus-mediated HSV-tk gene transfer under the driving of KDR promoter targeting the tumor vasculature.
Our in vivo results revealed that treatment with AdKDR-tk targeted therapy could inhibit visualization and shrink the tumor volume. Immuohistochemical (IHC) analysis of the subcutaneously implanted HCC tumors found that the microvessel density and tumor-associated endothelial cells were decreased with increased apoptosis of tumor cells. And, the CD31-positive blood vessels were also decreased compared with the normal control group. That would support the hypothesis that AdKDR-tk affects tumor growth primarily through anti-angiogenesis.
Although our studies was based on a mouse model, we expect AdKDR-tk motif to target human vasculature as well, for the HCC phage has been shown to bind to blood vessels of human tumors. Thus, the recombinant adenovirus is potentially suitable for tumor targeting in patients. Assessment of microvessel density using IHC staining for specific endothelial cell markers such as CD31 has been shown to provide prognostic information independent of conventional pathological parameters in HCC patients.
In conclusion, the targeting of the vasculature of diseased organs could be the basis of a new pharmacological approach for the treatment of malignancies by taking advantage of formulations that delivery cytotoxic drugs to the blood vessels located specifically at sites of disease. This should improve efficacy and reduce side effects. Anti-angiogenic therapy has also been proven to be a rational approach in the treatment of solid tumors. Selective delivery of the suicide gene into tumor endothelial cells and target its expression to abrogate tumor vasculature may be a good modality for tumor therapy. Antiangiogenic therapy holds the promise of providing an effective novel treatment for HCC, which is of great clinical significance as there is no effective systemic therapy available for HCC.
S- Editor Liu Y L- Editor Ma JY E- Editor Wang HF
| 1. | Butterfield LH, Ribas A. Immunotherapy of hepatocellular carcinoma. Expert Opin Biol Ther. 2002;2:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Palmer DH, Hussain SA, Johnson PJ. Gene- and immunotherapy for hepatocellular carcinoma. Expert Opin Biol Ther. 2005;5:507-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Mohr L. Hepatocellular carcinoma: novel therapeutic approaches. Praxis (Bern 1994). 2007;96:553-562. [PubMed] |
| 4. | Saharinen P, Alitalo K. Double target for tumor mass destruction. J Clin Invest. 2003;111:1277-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Iavarone M, Lampertico P, Iannuzzi F, Manenti E, Donato MF, Arosio E, Bertolini F, Primignani M, Sangiovanni A, Colombo M. Increased expression of vascular endothelial growth factor in small hepatocellular carcinoma. J Viral Hepat. 2007;14:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Lee TK, Poon RT, Yuen AP, Man K, Yang ZF, Guan XY, Fan ST. Rac activation is associated with hepatocellular carcinoma metastasis by up-regulation of vascular endothelial growth factor expression. Clin Cancer Res. 2006;12:5082-5089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Rehman S, Jayson GC. Molecular imaging of antiangiogenic agents. Oncologist. 2005;10:92-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | McCabe CJ, Boelaert K, Tannahill LA, Heaney AP, Stratford AL, Khaira JS, Hussain S, Sheppard MC, Franklyn JA, Gittoes NJ. Vascular endothelial growth factor, its receptor KDR/Flk-1, and pituitary tumor transforming gene in pituitary tumors. J Clin Endocrinol Metab. 2002;87:4238-4244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 136] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Shirakawa T, Gotoh A, Wada Y, Kamidono S, Ko SC, Kao C, Gardner TA, Chung LW. Tissue-specific promoters in gene therapy for the treatment of prostate cancer. Mol Urol. 2000;4:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Sassa Y, Hata Y, Aiello LP, Taniguchi Y, Kohno K, Ishibashi T. Bifunctional properties of peroxisome proliferator-activated receptor gamma1 in KDR gene regulation mediated via interaction with both Sp1 and Sp3. Diabetes. 2004;53:1222-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Szary J, Kalita K, Przybyszewska M, Dus D, Kieda C, Janik P, Szala S. KDR promoter can transcriptionally target cytosine deaminase suicide gene to cancer cells of nonendothelial origin. Anticancer Res. 2001;21:3471-3475. [PubMed] |
| 12. | Shen BQ, Lee DY, Gerber HP, Keyt BA, Ferrara N, Zioncheck TF. Homologous up-regulation of KDR/Flk-1 receptor expression by vascular endothelial growth factor in vitro. J Biol Chem. 1998;273:29979-29985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 154] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Yang WY, Huang ZH, Lin LJ, Li Z, Yu JL, Song HJ, Qian Y, Che XY. Kinase domain insert containing receptor promoter controlled suicide gene system selectively kills human umbilical vein endothelial cells. World J Gastroenterol. 2006;12:5331-5335. [PubMed] |
| 14. | He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509-2514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2861] [Cited by in RCA: 3044] [Article Influence: 112.7] [Reference Citation Analysis (0)] |
| 15. | Wiedswang G, Borgen E, Kåresen R, Qvist H, Janbu J, Kvalheim G, Nesland JM, Naume B. Isolated tumor cells in bone marrow three years after diagnosis in disease-free breast cancer patients predict unfavorable clinical outcome. Clin Cancer Res. 2004;10:5342-5348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Poon RT, Ng IO, Lau C, Yu WC, Yang ZF, Fan ST, Wong J. Tumor microvessel density as a predictor of recurrence after resection of hepatocellular carcinoma: a prospective study. J Clin Oncol. 2002;20:1775-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Zhang ZL, Liu ZS, Sun Q. Anti-tumor effect of thalidomide and paclitaxel on hepatocellular carcinoma in nude mice. Chin Med J (Engl). 2005;118:1688-1694. [PubMed] |
| 18. | Hernandez-Alcoceba R, Sangro B, Prieto J. Gene therapy of liver cancer. World J Gastroenterol. 2006;12:6085-6097. [PubMed] |
| 19. | Ribatti D, Vacca A, Nico B, Sansonno D, Dammacco F. Angiogenesis and anti-angiogenesis in hepatocellular carcinoma. Cancer Treat Rev. 2006;32:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Tandle A, Blazer DG, Libutti SK. Antiangiogenic gene therapy of cancer: recent developments. J Transl Med. 2004;2:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Patterson C, Wu Y, Lee ME, DeVault JD, Runge MS, Haber E. Nuclear protein interactions with the human KDR/flk-1 promoter in vivo. Regulation of Sp1 binding is associated with cell type-specific expression. J Biol Chem. 1997;272:8410-8416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Patterson C, Perrella MA, Hsieh CM, Yoshizumi M, Lee ME, Haber E. Cloning and functional analysis of the promoter for KDR/flk-1, a receptor for vascular endothelial growth factor. J Biol Chem. 1995;270:23111-23118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 138] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Jaggar RT, Chan HY, Harris AL, Bicknell R. Endothelial cell-specific expression of tumor necrosis factor-alpha from the KDR or E-selectin promoters following retroviral delivery. Hum Gene Ther. 1997;8:2239-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Mavria G, Harrington KJ, Marshall CJ, Porter CD. In vivo efficacy of HSV-TK transcriptionally targeted to the tumour vasculature is augmented by combination with cytotoxic chemotherapy. J Gene Med. 2005;7:263-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Wang J, Lu XX, Chen DZ, Li SF, Zhang LS. Herpes simplex virus thymidine kinase and ganciclovir suicide gene therapy for human pancreatic cancer. World J Gastroenterol. 2004;10:400-403. [PubMed] |
| 26. | Davidoff AM, Ng CY, Zhang Y, Streck CJ, Mabry SJ, Barton SH, Baudino T, Zhou J, Kerbel RS, Vanin EF. Careful decoy receptor titering is required to inhibit tumor angiogenesis while avoiding adversely altering VEGF bioavailability. Mol Ther. 2005;11:300-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Mizuguchi H, Hayakawa T. Targeted adenovirus vectors. Hum Gene Ther. 2004;15:1034-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |