Published online Jul 28, 2007. doi: 10.3748/wjg.v13.i28.3900
Revised: March 15, 2007
Accepted: March 21, 2007
Published online: July 28, 2007
We present a case of a 25-year-old female with diagnosed familial adenomatous polyposis and elevated carcinoembryonic antigen with negative family history. The suspicion of Gardner's syndrome was raised because extirpation of an osteoma of the left temporo-occipital region was made 10 years ago. Restorative procto-colectomy and ileal pouch anal anastomosis was made but histology delineated adenocarcinoma of the rectum (Dukes C stage). We conclude that cranial osteomas often precede gastrointestinal manifestations of familial adenomatous polyposis or Gardner's syndrome and such patients should be evaluated with genetic testing followed by colonoscopy if results are positive to prevent the development of colorectal carcinoma. If the diagnosis is positive all family members should be evaluated for familial adenomatous polyposis.
- Citation: Smud D, Augustin G, Kekez T, Kinda E, Majerovic M, Jelincic Z. Gardner's syndrome: Genetic testing and colonoscopy are indicated in adolescents and young adults with cranial osteomas: A case report. World J Gastroenterol 2007; 13(28): 3900-3903
- URL: https://www.wjgnet.com/1007-9327/full/v13/i28/3900.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i28.3900
Familial adenomatous polyposis (FAP)[1] and Gardner’s syndrome (GS)[2] were originally described as two different syndromes. The incidence of FAP is between 1 in 8300 and 1 in 14 025 live births affecting both genders equally, with a uniform worldwide distribution[3]. The incidence of GS is lower and is characterized by Gardner’s triad: intestinal polyposis and various bone and soft-tissue tumors, including osteomas, epidermal inclusion cysts, lipomas, fibromas, and desmoid fibromatoses[4-8]. Congenital hypertrophy of the retinal pigmented epithelium (CHRPE)[9], dental malformations[10], benign cystic lung tumours[11], mesenteric fibromatosis, dental abnormalities, gastric polyps, duodenal polyps, lymphoid hyperplasia of the terminal ileum and ileal adenomas represent facultative signs. If left unchecked, patients with GS inevitably develop intestinal carcinoma at a much younger age than those with sporadic intestinal carcinoma[12]. It is important, therefore, to identify GS early. Typically, the soft-tissue lesions occur first, alerting the clinician to the possibility of GS, because they are often numerous, superficial, and occur before the development of intestinal polyps. Because of variable expression of adenomatous polyposis coli (APC) gene mutations associated with GS, a wide range of phenotypes are observed clinically, with some patients having few soft-tissue lesions. The presence of desmoid fibromatoses, normally uncommon in young patients, should signal the presence of underlying GS[4-7]. The clinical spectrum of the disease presentation is variable and often diagnosis is delayed, despite the presence of clues for a significant amount of time.
We present the case of a 25-year-old girl with Gardner’s syndrome that was diagnosed due to the finding of FAP and cranial vault osteoma resected 10 years before the diagnosis of FAP.
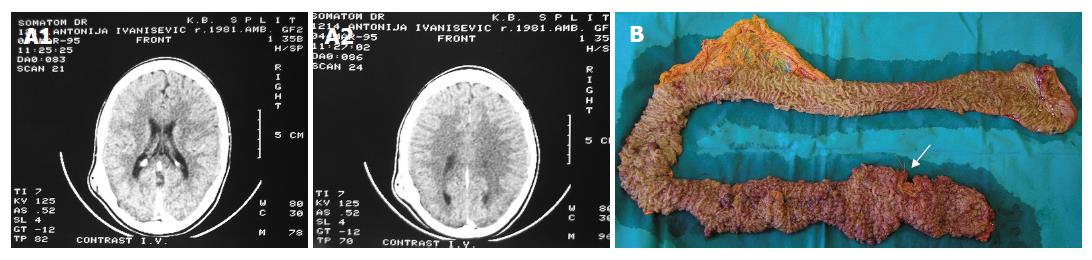
A 25-year-old female presented with abdominal cramps, especially after defecation, lasting for 10 years. From August 2005 abdominal pain was more severe, occasionally with blood in the stool. In August 2006 she was examined by the gastroenterologist. Colonoscopy delineated numerous polyps carpeting the entire colon and rectum, mostly sigmoid colon and rectum which was consistent with the diagnosis of FAP. Polypectomy of 4 polyps showed low grade dysplasia. Carcinoembryonic antigen was 10.38 ng/mL, the normal values being < 3.4 ng/mL. The clinical suspicion of GS was raised because extir-pation of an osteoma of the left temporooccipital region with osteoplastic craniotomy was made in 1995. That lump was present from the early childhood but in 1995 it grew larger and then she sought medical attention. Skull X-ray showed bone thickening and then CT showed 4 cm bone thickening without intracranial extension (intact inner table). Soft tissue thickening above this lesion was also evident (Figure 1A). The diagnosis of cancellous, trabecular type osteoma was confirmed histologically. No family history of colonic carcinoma or polyposis was found. She has two sisters who recall that she is the only sister who have had an abdominal cramps from the early childhood. Esophagogastroduodenoscopy and small bowel follow through were normal. The next step was a restorative proctocolectomy with ileal pouch anal anastomosis (RPC/IPAA) and mucosectomy. One suspicious transmural lesion was marked with a stitch for more detailed examination (Figure 1B). Pathology of the colon specimen confirmed the diagnosis of FAP. Colonic polyps were tubular adenomas with low grade dysplasia. Part of the rectum marked with the stitch was adenocarcinoma (Dukes C stage). 5-FU/leucovorin based chemotherapy was initiated after 3 wk because there were no postoperative complications. On discharge, it was recommended that all first-degree family members should be evaluated for FAP.
GS is considered a variant of FAP, in which certain extracolonic manifestations (e.g., osteomas and fibromas) develop. GS is caused by truncating mutations in a portion of the APC gene (codons 1403 and 1578) that differs from classic FAP (codons 169-1600), attenuated FAP (amino terminal to codon 157), and congenital hypertrophy of the retinal pigmented epithelium (codons 463-1387)[13]. Nonetheless, there is evidence that even patients with identical mutations may have different phenotypic expressions for reasons that are not clear[13]. The majority of individuals have a family history of this pathology, but 25% of patients can present with a new dominant mutation (de novo mutation) and be the first member of the family affected[14]. These patients are generally not under medical survaillance before they have bowel symptoms, and 67% of them will have developed colorectal cancer[15]. In 100% of all untreated patients, cancer of the large intestine develops before the age of 40. Hence, prophylactic colectomy is indicated[16,17], although desmoid tumors of the mesenteric and abdominal wall may develop after surgery[18].
The frequency of osteomas noticeable by sight or palpation after clinical examination are found in 21%-24% of polyposis patients from families with GS[19,20]. The majority of osteomas remain occult; the incidence in the general population is 0.014%-0.43%. The incidence is higher in female patients, predominantly in the 2nd and 3rd decades of life and is rare in puberty[21,22]. Cranial vault osteomas are less frequent than skull base osteomas and can be either enostotic (inner table) or exostotic (outer table)[23,24]. Mandibular osteomas are the commonest and largest[25-27]. The CT is the best imaging technique for the diagnosis of an osteoma. Histologically, the osteomas are normally formed by trabeculas of bone laminated with fibroadipose tissue. In the case of enostosis, these consist of compact islets of mature laminated bone[28]. Osteomas in the facial bones and cranium are common in patients affected by GS in contrast to the general population. Osteomas often precede the diagnosis of FAP, the fact that is important for early detection of these patients[27].
Genetic testing is the most efficient mode of identifying gene carriers in a FAP relative. In cases where the exact sequence of a mutation is known in one family member, direct sequencing directed at the region of the APC gene thought to be affected has been shown to be 90% cost effective and accurate[29]. Linkage analysis to markers on chromosome 5q, protein truncation testing, direct sequencing, conformation-sensitive gel electrophoresis, and single-strand, conformation-sensitive gel electrophoresis all have accuracies 70%-90%[30]. Splice site defects or gross genomic alterations may have been underestimated by the older testing methods and require cDNA screening and gene rearrangement testing[31]. Genetic risk assessment should precede the initiation of regular endoscopic screening[32]. Screening colonoscopy should begin at age 10-12 years for patients who are known to have APC mutations[33].
Congenital hypertrophy of the retinal pigment epithelium (CHRPE) refers to the presence of characteristic pigmented fundus lesions that occur in 70%-80% of patients with FAP[9]. These ophthalmic manifestations are usually present at birth, largely preceding the development of intestinal polyposis, and are asymptomatic with no malignant potential. They are specific to FAP, as opposed to other hereditary or sporadic colonic cancers[34]. The diagnostic criteria with the highest specificity/sensitivity for CHRPE include the detection of four small pigmented lesions, or two lesions of which one is large (> 25% of disc surface), using bilateral lens fundoscopic examination[35]. The presence of multiple bilateral lesions appears to be a highly specific marker for FAP (95%-100% specificity)[36]. This makes ophthalmological examination an attractive noninvasive and early diagnostic test for at-risk family members, aside from genetic testing.
In conclusion, we presented a case of GS with a typical clinical presentation, unfortunately unrecognized in the early stage. Cranial vault osteoma preceded significant gastrointestinal manifestations for 10 years. Successful RPC/IPAA was made with excellent immediate postoperative results with early institution of chemotherapy. According to the oncologic principles, the RPC/IPAA should not be performed in cases of histologically confirmed carcinoma because postoperative complications associated with IPAA would lead to significant delay in institution of chemotherapy. Seventy-six to 93% of patients with FAP having no clinical signs of Gardner’s syndrome have osteomas[37,38]. The rarity of the osteomas, their presentation at the same age as FAP and their often earlier presentation than gastrointestinal symptomatology in patients with Gardner’s syndrome should lead to adoption of the rule that all diagnosed cranial osteomas should be followed by ophthalmic and/or genetic testing for FAP followed by colonoscopy if the results are positive. With the confirmed diagnosis of FAP or Gardner’s syndrome, endoscopic evaluation should include stomach, duodenum and small bowel to prevent the development of adeno-carcinoma sequence in these regions. Biopsies of abnormal plaques of tissue or random biopsies of duodenal mucosa in patients with macroscopically normal appearances should be taken to allow the Spigelman stage to be determined, and the findings at each endoscopy used to stratify the patients’ risk, and determine the surveillance interval and management[39,40]. The vast majority of gastric polyps are fundic gland polyps which are hamartomas that harbor little if any malignant potential. At present there are insufficient data to recommend that management decisions regarding upper gastrointestinal neoplasia in FAP guided by mutation status. Proposed algorithms for screening probands and unaffected first-degree relatives with FAP are made by Galiatsatos et al[41]. Although heightened awareness, endoscopic surveillance, and the establishment of polyposis registries have successfully decreased the incidence and mortality from colorectal carcinoma, the challenge now lies in determining the optimal screening and therapeutic modalities for associated extracolonic malignancies that are consequently becoming more prominent. After prophylactic colectomy, FAP patients may still die from rectal cancer (if an ileorectal anastomosis was performed or cancer occurs in the rectal cuff remnants), from desmoid tumors, duodenal cancers, or from other uncommon complications[42,43]. These patients require life-long observation, medical (including nutrition and vitamin supplements) and emotional support.
S- Editor Zhu LH L- Editor Wang XL E- Editor Liu Y
| 2. | Garder EJ, Richards RC. Multiple cutaneous and subcutaneous lesions occurring simultaneously with hereditary polyposis and osteomatosis. Am J Hum Genet. 1953;5:139-147. [PubMed] |
| 3. | Wennstrom J, Pierce ER, McKusick VA. Hereditary benign and malignant lesions of the large bowel. Cancer. 1974;34:suppl: 850-uppl: 857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Garder EJ. Follow-up study of a family group exhibiting dominant inheritance for a syndrome including intestinal polyps, osteomas, fibromas and epidermal cysts. Am J Hum Genet. 1962;14:376-390. [PubMed] |
| 5. | Gorlin RJ, Chaudhary AP. Multiple osteomatosis, fibromas, lipomas and fibrosarcomas of the skin and mesentery, epidermoid inclusion cysts of the skin, leiomyomas and multiple intestinal polyposis: a heritable disorder of connective tissue. N Engl J Med. 1960;263:1151-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 59] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Pierce ER, Weisbord T, McKusick VA. Gardner's syndrome: formal genetics and statistical analysis of a large Canadian kindred. Clin Genet. 1970;1:65-80. [RCA] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Staley CJ. Gardner's syndrome: simultaneous occurrence of polyposis coli, osteomatosis, and soft tissue tumors. Arch Surg. 1961;82:420-422. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Weary PE, Linthicum A, Cawley EP, Coleman CC Jr, Graham GF. Garder's syndrome. A Family group study and review. Arch Dermatol. 1964;90:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Traboulsi EI, Krush AJ, Gardner EJ, Booker SV, Offerhaus GJ, Yardley JH, Hamilton SR, Luk GD, Giardiello FM, Welsh SB. Prevalence and importance of pigmented ocular fundus lesions in Gardner's syndrome. N Engl J Med. 1987;316:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 148] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Chang CH, Piatt ED, Thomas KE, Watne AL. Bone abnormalities in Gardner's syndrome. Am J Roentgenol Radium Ther Nucl Med. 1968;103:645-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Török L, Fazekas A, Domján L, Budai S, Kása M. Gardner syndrome. Hautarzt. 1990;41:83-86. [PubMed] |
| 12. | Bussey HJR. Historical developments in familial adenomatous polyposis. Herrera L, editor. New York: Alan R. Liss 1990; 1-7. |
| 13. | Vogelstein B, Kinzler KW. Colorectal tumors. The Genetic Basis of Human Cancer. New York: McGraw-Hill 1998; 565-587. |
| 14. | Bisgaard ML, Fenger K, Bülow S, Niebuhr E, Mohr J. Familial adenomatous polyposis (FAP): frequency, penetrance, and mutation rate. Hum Mutat. 1994;3:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 294] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Bülow S. Results of national registration of familial adenomatous polyposis. Gut. 2003;52:742-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 16. | Watne AL, Core SK, Carrier JM. Gardner's syndrome. Surg Gynecol Obstet. 1975;141:53-56. [PubMed] |
| 17. | Gingold BS, Jagelman D, Turnbull RB. Surgical management of familial polyposis and Gardner's syndrome. Am J Surg. 1979;137:54-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Soravia C, Berk T, McLeod RS, Cohen Z. Desmoid disease in patients with familial adenomatous polyposis. Dis Colon Rectum. 2000;43:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Leppard B, Bussey HJ. Epidermoid cysts, polyposis coli and Gardner's syndrome. Br J Surg. 1975;62:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 48] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Bisgaard ML, Bülow S. Familial adenomatous polyposis (FAP): genotype correlation to FAP phenotype with osteomas and sebaceous cysts. Am J Med Genet A. 2006;140:200-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Gupta OP, Samant HC. Osteoma of the mastoid. Laryngoscope. 1972;82:172-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Denia A, Perez F, Canalis RR, Graham MD. Extracanalicular osteomas of the temporal bone. Arch Otolaryngol. 1979;105:706-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Eppley BL, Kim W, Sadove AM. Large osteomas of the cranial vault. J Craniofac Surg. 2003;14:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Haddad FS, Haddad GF, Zaatari G. Cranial osteomas: their classification and management. Report on a giant osteoma and review of the literature. Surg Neurol. 1997;48:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Ziter FM. Roentgenographic findings in gardner's syndrome. JAMA. 1965;192:1000-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Plenk HP, Gardner EJ. Osteomatosis (leontiasis ossea): hereditary disease of membranous bone formation associated in one family with polyposis of the colon. Radiology. 1954;62:830-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Jones EL, Cornell WP. Gardner's syndrome; review of the literature and report on a family. Arch Surg. 1966;92:287-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Sayan NB, Uçok C, Karasu HA, Günhan O. Peripheral osteoma of the oral and maxillofacial region: a study of 35 new cases. J Oral Maxillofac Surg. 2002;60:1299-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | Cromwell DM, Moore RD, Brensinger JD, Petersen GM, Bass EB, Giardiello FM. Cost analysis of alternative approaches to colorectal screening in familial adenomatous polyposis. Gastroenterology. 1998;114:893-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Powell SM, Petersen GM, Krush AJ, Booker S, Jen J, Giardiello FM, Hamilton SR, Vogelstein B, Kinzler KW. Molecular diagnosis of familial adenomatous polyposis. N Engl J Med. 1993;329:1982-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 448] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 31. | Mihalatos M, Apessos A, Dauwerse H, Velissariou V, Psychias A, Koliopanos A, Petropoulos K, Triantafillidis JK, Danielidis I, Fountzilas G. Rare mutations predisposing to familial adenomatous polyposis in Greek FAP patients. BMC Cancer. 2005;5:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Mak T, Speake D, Lalloo F, Hill J, Evans DG. Familial colorectal cancer referral to regional genetics department--a single centre experience. Fam Cancer. 2007;6:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Cruz-Correa M, Giardiello FM. Diagnosis and management of hereditary colon cancer. Gastroenterol Clin North Am. 2002;31:537-549, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Traboulsi EI, Maumenee IH, Krush AJ, Giardiello FM, Levin LS, Hamilton SR. Pigmented ocular fundus lesions in the inherited gastrointestinal polyposis syndromes and in hereditary nonpolyposis colorectal cancer. Ophthalmology. 1988;95:964-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Tiret A, Taiel-Sartral M, Tiret E, Laroche L. Diagnostic value of fundus examination in familial adenomatous polyposis. Br J Ophthalmol. 1997;81:755-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Morton DG, Gibson J, Macdonald F, Brown R, Haydon J, Cullen R, Rindl M, Hulten M, Neoptolemos JP, Keighley MR. Role of congenital hypertrophy of the retinal pigment epithelium in the predictive diagnosis of familial adenomatous polyposis. Br J Surg. 1992;79:689-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Utsunomiya J, Nakamura T. The occult osteomatous changes in the mandible in patients with familial polyposis coli. Br J Surg. 1975;62:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 98] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Bülow S, Søndergaard JO, Witt I, Larsen E, Tetens G. Mandibular osteomas in familial polyposis coli. Dis Colon Rectum. 1984;27:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;2:783-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 473] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 40. | Iida M, Aoyagi K, Fujimura Y, Matsumoto T, Hizawa K, Nakamura S. Nonpolypoid adenomas of the duodenum in patients with familial adenomatous polyposis (Gardner's syndrome). Gastrointest Endosc. 1996;44:305-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Galiatsatos P, Foulkes WD. Familial adenomatous polyposis. Am J Gastroenterol. 2006;101:385-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 388] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 42. | Galle TS, Juel K, Bülow S. Causes of death in familial adenomatous polyposis. Scand J Gastroenterol. 1999;34:808-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Burke CA, Beck GJ, Church JM, van Stolk RU. The natural history of untreated duodenal and ampullary adenomas in patients with familial adenomatous polyposis followed in an endoscopic surveillance program. Gastrointest Endosc. 1999;49:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 130] [Article Influence: 5.0] [Reference Citation Analysis (0)] |