Published online Jul 28, 2007. doi: 10.3748/wjg.v13.i28.3883
Revised: April 23, 2007
Accepted: April 30, 2007
Published online: July 28, 2007
AIM: To explore the expression of BAG1 and tissue inhibitor of metalloproteinase 3 (TIMP3) in colon carcinoma and their correlation and clinicopathologic significance.
METHODS: SABC immunohistochemistry was used to detect the expression of BAG1 and TIMP3 in 80 colon carcinoma tissues and 20 normal colonic mucosa.
RESULTS: Positive rate of BAG1 in colon carcinoma tissue (80%) was notably higher compared to normal colonic mucosa (10%) (P < 0.05). However, no significant difference was observed in positive rate of TIMP3 in colon carcinoma tissue (43.75%) as compared with normal colonic mucosa (60%) (P > 0.05). Expression of BAG1 and TIMP3 was strongly associated with colon carcinoma differentiation, Duke’s staging, lymph node metastasis and survival rate (P < 0.05), but not associated with gender and age. Moreover, BAG1 expression was not correlated with TIMP3.
CONCLUSION: Our results suggest that over-expression of BAG1 or attenuated expression of TIMP3 may play an important role in genesis and development of colon carcinoma. The protein expression levels of BAG1 and TIMP3 are related to the malignant degree, infiltration and metastasis of colon carcinoma. BAG1 and TIMP3 might be new biological parameters in predicting invasion and metastasis of colon carcinoma.
- Citation: Bai YX, Yi JL, Li JF, Sui H. Clinicopathologic significance of BAG1 and TIMP3 expression in colon carcinoma. World J Gastroenterol 2007; 13(28): 3883-3885
- URL: https://www.wjgnet.com/1007-9327/full/v13/i28/3883.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i28.3883
At present, the mechanism of genesis and development of colon carcinoma is still under investigation. It is generally accepted that carcinogenesis is a complicated process that involves multistages, multisteps and polygene transformation. Of these, apoptosis is an important phenomenon responsible for tumor genesis. BAG1 is a newly discovered anti-apoptosis gene. Its protein has been proven to be a multifunctional binding gene, which can enhance the ability to resist bcl-2-mediated apoptosis. TIMP3, an anti-oncogene which was discovered in recent years, is called tissue inhibitor of metalloproteinase 3. The study was designed to investigate the expression of BAG1 and TIMP3 in colon carcinoma tissues, and to explore their association with genesis, development and prognosis of colon carcinoma.
Samples were collected from surgical specimens of 80 patients (47 males and 33 females, average age 56.3 years) with pathologically proven colon carcinoma who had undergone surgery in Tumor Hospital of Harbin Medical University from January 1999 to January 2000. No patients had received any type of preoperative anti-tumor treatments. Of those 80 samples, 28 were well-differentiated, 37 moderately differentiated and 15 poorly differentiated adenocarcinomas. According to Duke’s staging classification, 24 cases were assigned to stage A, 22 cases assigned to stage B, 21 cases assigned to stage C, and 13 cases assigned to stage D. Of the 80 cases, 32 had lymph node metastasis. In addition, 20 specimens from normal colonic mucosa were selected as control group. Both rabbit anti-human BAG1 and TIMP3 polyclonal antibodies were purchased from Wuhan Boster Biotechnical Company and were diluted to 1:100.
All samples were fixed with 100 mL/L formalin and embedded in paraffin. Each paraffin block was cut into 4-μm thick sections. SABC immunohistochemistry was performed according to the manufacturer’s instructions to detect the gene expression of BAG1 and TIMP3. Briefly, the tissue sections were deparaffinized in xylene at 37°C for 20 min. Endogenous peroxide was blocked by incubating the slides with 30 mL/L H2O2 for 10 min at 37°C. Sections were incubated with primary antibodies of BAG1 and TIMP3 at 4°C overnight, respectively. Staining was visualized with DAB for 10 min at room temperature. Finally, the sections were counterstained for nuclei by hematoxylin solution. Five visual fields were randomly observed in each section under microscope (10 × 40 magnification) and at least 100 cells were counted in each field.
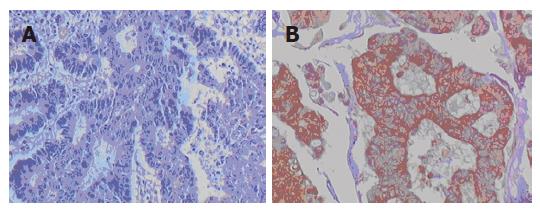
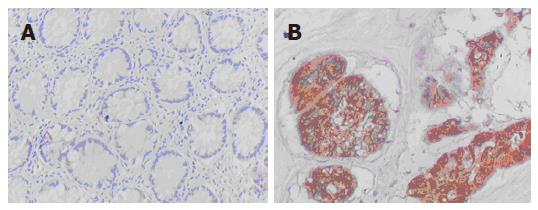
Positive staining of BAG1 appeared as buffy grains in the nucleus and cytoplasm and whereas that of TIMP3 appeared as buffy grains in intracytoplasm observed. Positive expression was considered when cells were stained more than 10%.
In this study, a semi-quantitative method was adopted to judge the result. The color intensity of tumor cells were graded on a scale of 0 to 3 as follows: no = 0, mild = 1, moderate = 2 and severe = 3. In addition, according to the percentage of positive cells, the color density of tumor cells were graded on a scale of 0 to 3 as follows: ≤ 25% = 0, 26%-50% = 1, 51%-75% = 2; and > 75% = 3. The staining result was evaluated by grade product of color intensity and density as follows: 0 = negative (-), 1-4 = weakly positive (+), and > 4 = positive (++). All specimens were evaluated by two pathologists without any prior knowledge of the clinical data of the patients.
Gene expression was considered attenuated or absent when grade product ranged from 0 to 4, and considered normal or positive when grade product was higher than 4.
Chi-square test was used to compare the positive expression rates of BAG1 and TIMP3 in cancer and normal tissues. Pearson’s correlation coefficient (r) was used to analyze the relation between BAG1 and TIMP3.
Expression of BAG1 and TIMP3 in colon carcinoma and normal colon tissues is shown in Table 1. Positive expression rate of BAG1 and TIMP3 was not correlated with age, and gender of the patients, but was associated with degree of tumor differentiation, Duke’s staging, metastasis and survival (P < 0.05) (Table 2). Interestingly, the poorer the cancer differentiation, the more advanced the Duke's staging and the more lymph node metastases, the higher was the BAG1 expression, but the lower was the TIMP3 expression. Along with these features, the 5-year survival rate was low in those patients.
| Tissue type | n | BAG1 | TIMP3 |
| Positive number (%) | Positive number (%) | ||
| Benign | 20 | 2 (10.00) | 12 (60.00) |
| Malignant | 80 | 64 (80.00)a | 35 (43.75) |
| Clinico-pathologicindex | n | BAG1 | TIMP3 | |||
| Positivenumber | χ2 value | Positivenumber | χ2 value | |||
| Age | ≥ 50 | 59 | 50 | 2.13 | 29 | 2.67 |
| < 50 | 21 | 14 | 6 | |||
| Sex | Male | 47 | 38 | 0.05 | 17 | 0.007 |
| Female | 33 | 26 | 18 | |||
| Stage | Duke’s A | 24 | 10 | 11.35 | 16 | 7.9 |
| Duke’s B | 22 | 18a | 9a | |||
| Duke’s C | 21 | 20a | 7a | |||
| Duke’s D | 13 | 12a | 3a | |||
| Differen | Well | 28 | 17 | 9.92 | 19 | 10.97 |
| -tiation | Moderate | 37 | 33a | 13a | ||
| Poorly | 15 | 14a | 3a | |||
| Lymphonode | Yes | 32 | 30 | 6.3 | 5 | 17.14 |
| metastasis | No | 48 | 34a | 30a | ||
| Survival | ≥ 5 yr | 38 | 26 | 6.07 | 27 | 21.92 |
| < 5 yr | 42 | 38a | 8a | |||
Figures 1 and 2 depict the protein expression of BAG1 and TIMP3, respectively. As shown in Table 3, there was no significant relationship between BAG1 and TIMP3 expression.
| Detection index | BAG1 | χ2 value | P value r value |
| Positive Negative | |||
| TIMP3 Positive | 25 10 | 2.86 | P > 0.05 0.0856 |
| Negative | 39 6 |
Human BAG1 gene is located at chromosome 9p12 and it can encode a multifunctional protein. Recent studies showed that BAG1 was scarcely expressed in normal tissue, but highly expressed in breast carcinoma, lung cancer, thyroid cancer, endometrial cancer and gastrointestinal tract cancer[1-6]. BAG1 protein has been found to be strongly positively associated with proliferation, infiltration and metastasis of tumor cells[7,8]. BAG1 can also interact with many target molecules, such as HSP70, RAF-1, Bcl-2 and nuclear hormone receptor, to regulate the growth and survival of tumor cells[9]. Down-regulation of BAG1 expression can enhance apoptosis and hinder tumor from genesis and development. However, over-expression of BAG1 can inhibit apoptosis caused by radiotherapy, chemotherapy, heat shock, activation of dead molecules on cell-surface, etc so as to stimulate the genesis and development of tumor.
Our data revealed that the poorer the cancer differentiation, the more advanced the Duke's staging and the more lymph node metastases, the higher was the BAG1 expression, suggesting that high expression of BAG1 stimulates the development of colon carcinoma by inhibiting apoptosis and promoting tumor cell proliferation and metastasis. Up-regulation of BAG1 expression was associated with worse prognosis of the patients. Our results are consistent with previous studies[2,3,5] that high expression of BAG1 may be a new effective biologic index for predicting potency of metastasis and prognosis of colon carcinoma.
Human tissue inhibitor of metalloproteinase-3 (TIMP3) gene is located at chromosome 22 q12.3 and is a natural inhibitor for matrix metalloproteinase (MMP). TIMP3 can stimulate proliferation and transformation; thereby plays an important role in tumor angiogenesis, degradation of extracellular matrix and migration endotheliocyte. Moreover, TIMP3 can induce cells to undergo apoptosis[10]. Current research showed that attenuated expression of TIMP3 existed in many cancers, such as chorionic carcinoma, prostatic carcinoma, esophageal carcinoma, melanotic cancer[11-14].
In this study, no significant difference was observed in TIMP3 expression in colon carcinoma tissue as compared to normal colonic mucosa. With the poorer tumor differentiation, the advanced Duke’s staging and the emerge of lymph node metastasis, TIMP3 expression was decreased and patient’s prognosis was worse and worse. On the basis of these premises, we think TIMP3 may play an important role in tumor infiltration and metastasis instead of tumor genesis. These findings are similar to that reported by previous studies[14,15]. Thus, TIMP3 may be an important measure to inhibit growth and invasion of tumor by artificial induction.
Our study did not provide enough data to prove any correlation between BAG1 and TIMP3.
In conclusion, our findings reveals that high expression of BAG1 and attenuated or no expression of TIMP3 in colon carcinoma are strongly associated with the poor differentiation, advanced Duke’s staging, more lymph node metastasis, and low 5-year survival rate. BAG1 and TIMP3 might be new biological parameters in predicting invasion and metastasis of colon carcinoma.
S- Editor Liu Y L- Editor Kumar M E- Editor Wang HF
| 1. | Tang SC. BAG-1, an anti-apoptotic tumour marker. IUBMB Life. 2002;53:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Kikuchi R, Noguchi T, Takeno S, Funada Y, Moriyama H, Uchida Y. Nuclear BAG-1 expression reflects malignant potential in colorectal carcinomas. Br J Cancer. 2002;87:1136-1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Krajewska M, Turner BC, Shabaik A, Krajewski S, Reed JC. Expression of BAG-1 protein correlates with aggressive behavior of prostate cancers. Prostate. 2006;66:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Townsend PA, Dublin E, Hart IR, Kao RH, Hanby AM, Cutress RI, Poulsom R, Ryder K, Barnes DM, Packham G. BAG-i expression in human breast cancer: interrelationship between BAG-1 RNA, protein, HSC70 expression and clinico-pathological data. J Pathol. 2002;197:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Rorke S, Murphy S, Khalifa M, Chernenko G, Tang SC. Prognostic significance of BAG-1 expression in nonsmall cell lung cancer. Int J Cancer. 2001;95:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Moriyama T, Littell RD, Debernardo R, Oliva E, Lynch MP, Rueda BR, Duska LR. BAG-1 expression in normal and neoplastic endometrium. Gynecol Oncol. 2004;94:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Alberti S, Esser C, Höhfeld J. BAG-1--a nucleotide exchange factor of Hsc70 with multiple cellular functions. Cell Stress Chaperones. 2003;8:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Sirvent JJ, Aguilar MC, Olona M, Pelegrí A, Blázquez S, Gutiérrez C. Prognostic value of apoptosis in breast cancer (pT1-pT2). A TUNEL, p53, bcl-2, bag-1 and Bax immunohistochemical study. Histol Histopathol. 2004;19:759-770. [PubMed] |
| 9. | Barnes JD, Arhel NJ, Lee SS, Sharp A, Al-Okail M, Packham G, Hague A, Paraskeva C, Williams AC. Nuclear BAG-1 expression inhibits apoptosis in colorectal adenoma-derived epithelial cells. Apoptosis. 2005;10:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Deng X, Bhagat S, Dong Z, Mullins C, Chinni SR, Cher M. Tissue inhibitor of metalloproteinase-3 induces apoptosis in prostate cancer cells and confers increased sensitivity to paclitaxel. Eur J Cancer. 2006;42:3267-3273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Darnton SJ, Hardie LJ, Muc RS, Wild CP, Casson AG. Tissue inhibitor of metalloproteinase-3 (TIMP-3) gene is methylated in the development of esophageal adenocarcinoma: loss of expression correlates with poor prognosis. Int J Cancer. 2005;115:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Feng H, Cheung AN, Xue WC, Wang Y, Wang X, Fu S, Wang Q, Ngan HY, Tsao SW. Down-regulation and promoter methylation of tissue inhibitor of metalloproteinase 3 in choriocarcinoma. Gynecol Oncol. 2004;94:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Yegnasubramanian S, Kowalski J, Gonzalgo ML, Zahurak M, Piantadosi S, Walsh PC, Bova GS, De Marzo AM, Isaacs WB, Nelson WG. Hypermethylation of CpG islands in primary and metastatic human prostate cancer. Cancer Res. 2004;64:1975-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 367] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 14. | Bachman KE, Herman JG, Corn PG, Merlo A, Costello JF, Cavenee WK, Baylin SB, Graff JR. Methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene suggest a suppressor role in kidney, brain, and other human cancers. Cancer Res. 1999;59:798-802. [PubMed] |
| 15. | Kimura N, Nagasaka T, Murakami J, Sasamoto H, Murakami M, Tanaka N, Matsubara N. Methylation profiles of genes utilizing newly developed CpG island methylation microarray on colorectal cancer patients. Nucleic Acids Res. 2005;33:e46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |