Published online Jul 28, 2007. doi: 10.3748/wjg.v13.i28.3873
Revised: February 28, 2007
Accepted: March 28, 2007
Published online: July 28, 2007
AIM: To investigate the distribution and frequency of advanced polyps over eight years.
METHODS: 6424 colonoscopies were reviewed during the study period 1998 to 2005. The study period was subdivided into period I: 1998 to 2001 and period II: 2002-2005.
RESULTS: 1856 polyps (33% advanced polyps) and 328 CRCs were detected. The mean ages of the patients with advanced polyps and cancer were 69.2 ± 12.0 and 71.6 ± 13.8 years, respectively. Advanced polyps were mainly left sided (59.5%). Advanced polyps were found in patients ≤ 60 years from 17.7% in periodI to 26.3% in period II (P < 0.05), especially in male subjects ≤ 60 years (21.6% vs 31.6%, P < 0.05). Advanced tubulovillous polyps rose from 21.5% in period I to 29.5% in period II (P < 0.05). Whereas cancers in male patients ≤ 60 years were similar in both periods: 23.2% vs 16.5% (P > 0.05).
CONCLUSION: Advanced polyps increased significantly in the younger male group in the most recent period and there seems to be a shift towards a proximal location.
- Citation: Lam TJ, Wong BC, Mulder CJ, Peña AS, Hui WM, Lam SK, Chan AOO. Increasing prevalence of advanced colonic polyps in young patients undergoing colonoscopy in a referral academic hospital in Hong Kong. World J Gastroenterol 2007; 13(28): 3873-3877
- URL: https://www.wjgnet.com/1007-9327/full/v13/i28/3873.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i28.3873
Colorectal cancer (CRC) is one of the most common malignancies in the world. In Hong Kong, CRC is ranked as the second most common cancer regarding the incidence in men and women, whereas the mortality of colorectal cancer has a second position in women and third position in men[1].
A change in the epidemiology of CRC has been observed in the past decades. Firstly, there was proximal shift of CRC, reported by Axtell and Chiazze[2] and then confirmed by other studies[3-7]. Secondly, there was a change in the mean age of onset of CRC. While studies from western countries showed that CRC were increasing in young adults[8], studies from China[9], and in Japan[10] showed that the mean age of onset of CRC were increasing.
It is widely recognized that the majority of CRC arise from neoplastic polyps[11-14]. The concept of an adenoma-carcinoma sequence has been supported by pathological[15], molecular genetic[16,17] and cell biological[18] findings. In addition, it has been demonstrated that colonoscopic removal of adenomatous polyps was effective in reducing the incidence of CRC[19].
The prevalence of advanced polyps in Hong Kong is 12.5%[20]. This is quite a high prevalence when compared with other studies of the Chinese population. In Taiwan, Cheng et al[21] reported an advanced polyps’ prevalence of 1.3%, and Soon et al[22] a prevalence of 4.0%.
We aimed at investigating the change of distribution and the mean age of onset of colorectal polyps, if any, over the past eight years in a regional hospital. The study has implication in the future planning of screening strategies.
During the study period of January 1998 - July 2005, data was collected retrospectively from all colonoscopy reports made at the Queen Mary Hospital, Hong Kong. Queen Mary with its 1400 beds, is a teaching hospital of the medical school of the University of Hong Kong.
The data included the patient’s sex and age at the time of endoscopic examination. The size, location and the histology of each polyp were also recorded. Patients with personal or family history of colorectal cancer or polyps were not excluded in this study.
A polyp was defined as advanced if it was ≥ 10 mm in diameter, with a villous component, or had moderate to severe dysplasia. Invasive cancer was excluded in the definition. In this study polyps were considered right sided if they occurred at or proximal to the splenic flexure and left-sided if they occurred distal to the splenic flexure.
All the polyps were divided into two groups based on the year of the examination: period I was from 1998 to 2001; period II from 2002 to 2005. Chi-square test (Fisher’s Exact Test) was performed to compare the proportions. All P-values were two-tailed and statistical significance was taken as a P-value of less than 0.05. Analyses were performed with the statistical software SPSS version 11.0.
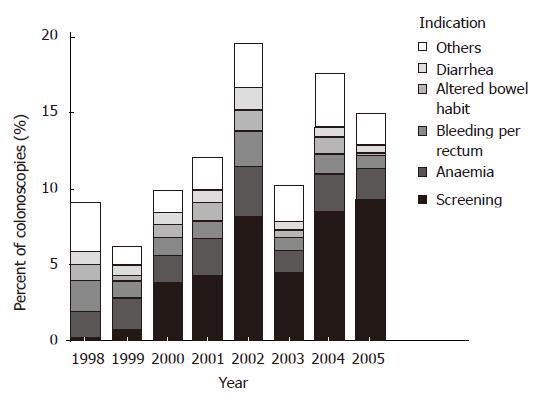
From January 1998 through July 2005, 6424 colonoscopies were performed. The indications for colonoscopy where polyps were found, were screening (24.5% and 49.2% respectively in period Iand period II, P < 0.001), anemia (22.0% vs 14.8%, P < 0.001), bleeding per rectum (14.3% vs 8.7%, P < 0.05), altered bowel habits (9.3% vs 4.7%, P < 0.001), diarrhea (8.0% vs 5.0%, P < 0.05) and others (22.0% vs 17.6%, P > 0.05). Figure 1 examines the indications for the colonoscopies by year. Despite the increased screening program in period II, the male to female ratio and the age of the population that has been screened stayed the same in both periods.
In total, 1856 polyps were detected in 1064 colono-scopies from 880 patients. The prevalence of polyps was higher in male subjects (63.5%). The mean age of the study population with polyps was 67 ± 12 (range 20-96) years. Polyps were mainly left sided (58.1%). Table 1 shows the histology of the polyps.
| No. of polyps | No. of advanced polyps | No. of patients | No. of patients with advanced polyps | |
| (n = 1856) | (n = 613) | (n = 880) | (n = 376) | |
| Villous component | 196 (10.5) | 196 (31.9) | 148 (18.8) | 148 (39.3) |
| Villous | 34 (1.8) | 34 (5.5) | 23 (2.6) | 23 (6.1) |
| Tubulovillous | 162 (8.7) | 162 (26.4) | 125 (14.2) | 125 (33.2) |
| Tubular | 1014 (54.6) | 416 (67.9) | 485 (55.1) | 227 (60.4) |
| Flat adenoma | 5 (0.3) | 1 (0.2) | 2 (0.2) | 1 (0.3) |
| Serrated adenoma | 6 (0.3) | 3 (0.3) | ||
| Hyperplastic | 339 (18.3) | 131 (14.9) | ||
| Other | 296 (16.0) | 111 (12.6) |
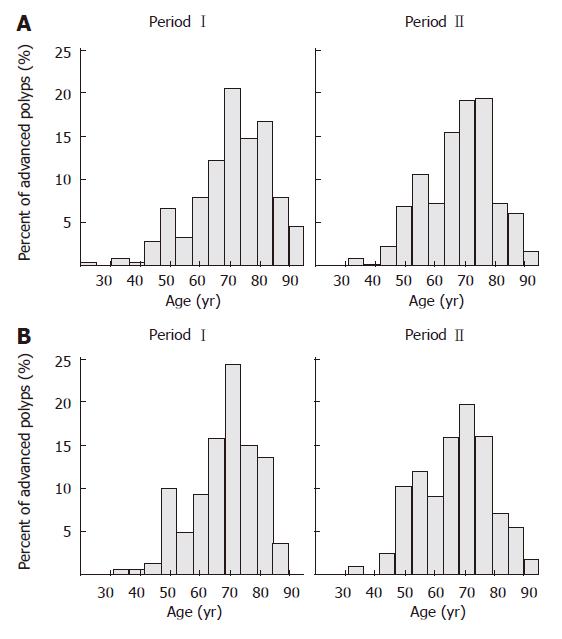
Among the 1856 polyps, 613 (33.0%) were advanced polyps from 376 patients in 440 colonoscopies. The mean age of the study population with advanced polyps was 69 ± 12 (range 22-91) years (Figure 2). The prevalence of polyps was also higher in male subjects (63%) The mean age of the male population with advanced polyps was 67 ± 12 (range 32-90) years. Advanced polyps were mainly left sided (59.5%). Among them, 137 (22.3%) polyps were isolated right-sided (Table 2).
| Period I1998-2001 | Period II2002-2005 | Total | |
| (n = 237) | (n = 376) | (n = 613) | |
| Location | |||
| Right | 46 (19.4) | 91 (24.2) | 137 (22.3) |
| Left | 115 (48.5) | 130 (34.6) | 245 (40.0) |
| Both | 76 (32.1) | 155 (41.2) | 231 (37.7) |
The polyps were sub-classified into 2 periods according to the year of the examination. The male subjects increased from 62.2% to 64.2% in period II (P > 0.05). In period II the prevalence of polyps increased in the group ≤ 60 years from 24.2% to 29.2% (P < 0.05), especially in the male group ≤ 60 years. It increased from 26.4% to 32.0% (P < 0.05).
When only advanced polyps were analyzed, it could be seen that the prevalence increased in the younger male group in period II from 21.6% to 31.6% (P < 0.05). In the younger female group advanced polyps increased from 12.2% to 16.7% (P > 0.05). After correcting the gender as confounder with the Mantel-Haeszel test, the P-value was still < 0.05 over the two periods for the younger population with advanced polyps (Table 3).
| Period I | Period II | Total | P-value | |
| 1998-2001 | 2002-2005 | |||
| Gender | ||||
| Male | 139 (58.6) | 244 (64.9) | 383 (62.5) | |
| Female | 98 (41.4) | 132 (35.1) | 230 (37.5) | P > 0.05 |
| Age (yr) | ||||
| ≤ 60 | 42 (17.7) | 99 (26.3) | 141 (23.0) | |
| > 60 | 195 (82.3) | 277 (73.7) | 472 (77.0) | P < 0.05 |
| Location | ||||
| Right | 85 (35.9) | 163 (43.4) | 248 (40.5) | |
| Left | 152 (64.1) | 213 (56.6) | 365 (59.5) | P > 0.05 |
| Tubulovillous | ||||
| Yes | 51 (21.5) | 111 (29.5) | 162 (26.4) | |
| No | 186 (78.5) | 265 (70.5) | 451 (73.6) | P < 0.05 |
It could also be seen that by screening the prevalence of advanced polyps increased from 23.5% to 31.4% (P < 0.05) between the periods (Table 4). Screening indication was not significant for the younger group between the two periods. The mean age of the younger age group with advanced polyps detected by screening was 68 ± 14 (range 33-88) vs 69 ± 11 (range 42-91) years in periodI and period II.
| Period I | Period II | Total no of CRCs | P-value | |
| 1998-2001 | 2002-2005 | |||
| (n = 183) | (n = 606) | (n = 789) | ||
| Advanced | ||||
| Yes | 43 (23.5) | 190 (31.4) | 233 (29.5) | |
| No | 140 (76.5) | 416 (68.6) | 556 (70.5) | P < 0.05 |
The proportion of advanced tubulovillous polyps rose from 21.5% in period I to 29.5% in period II (P < 0.05). The prevalence of tubulovillous polyps was higher in male subjects (66%). The mean age of the study population with tubulovillous polyps was 68 ± 12 (range 33-90) years. Among the tubulovillous polyps 79% had moderate to severe dysplasia (P < 0.05).
A total of 328 patients were diagnosed with colorectal cancer. The mean age of the 328 patients with cancer was 71 ± 13 (range 22-101) years. Of the 328 cancer lesions, 58 patients (17.7%) were ≤ 60 years. Among the 58 patients 34 (58.6%) were male subjects. The male patients with cancer reduced from 23.2% to 16.5% (P > 0.05) (Table 5).
| Period I | Period II | Total no of CRCs | P-value | |
| 1998-2001 | 2002-2005 | |||
| (n = 82) | (n = 91) | (n = 173) | ||
| Age (yr) | ||||
| ≤ 60 | 19 (23.2) | 15 (16.5) | 34 (19.7) | |
| > 60 | 63 (76.8) | 76 (83.5) | 139 (80.3) | P > 0.05 |
This study, which covered a period of 8 years, from 1998 to 2005, found 613 (33.0%) advanced polyps in a total of 1856 polyps. In particular, we observed that the prevalence of polyps increased in young male patients (26.4% to 32.0%), this is also true for the prevalence of advanced polyps (21.6% to 31.6%).
O’Connell et al[8] demonstrated an increase in CRC in young patients. This observation is further corroborated in our current study. However, this contrasts with the conclusion of Li and Gu[9]. They suggested an increased mean age of the colorectal cancer patient in China, despite no statistical significance. The controversial findings between O’Connell et al[8] and Li may reflect the influence of culture and dietary habits.
The present study shows a tubulovillous prevalence of 13.3% of the adenomatous polyps and that the proportion of advanced polyps with tubulovillous adenoma rose from 21.5% in period I to 29.5% in period II (P < 0.05). Although the prevalence of tubulovillous is greater in the second period, it is still low compared with the study of Khan et al[23]. These authors reported a prevalence of 26.2% of the adenomatous polyps. Isbister[24] and Smith et al[25] even showed a higher prevalence of tubulovillous as histology (32% and 30.2%, respectively). A possible explanation for this discrepancy is the difference in race, culture and diet. The prevalence of Khan and Smith are based on a Western population and the present study is based on the Chinese population of Hong Kong.
Nonetheless, there are some limitations in our study that should be considered when interpreting these findings. First, the included patients are a hospital-based population. The indications during period I and II were different. Therefore, a direct comparison of the polyp prevalence of the two periods may not be appropriate.
The second limitation is the lack of individual-level data. Therefore, it was not possible to adjust the risk factors of CRC, such as the influence of culture and socioeconomic differences in dietary habits, obesity, use of tobacco and/or alcohol, genetic risk factors and physical activity during the two periods[26-30].
Although the findings of this study do not mirror the prevalence rates of colorectal polyps in the general population, they do indicate that advanced polyps are detected at a younger age among men undergoing colonoscopy. Regarding the similar incidence of cancer in time, it is possible that colonoscopy is an effective way to prevent CRC in the younger males.
We thank the endoscopy nurses from the Department of Medicine, Queen Mary Hospital, Hong Kong, for their nursing assistance. TJ Lam also gratefully acknowledges the financial support of Tramedico BV Netherlands and AstraZeneca Netherlands who allowed her to perform the studies in Queen Mary Hospital in Hong Kong.
CRC is one of the most common malignancies in the world. In Hong Kong, CRC is ranked as the second most common cancer regarding the incidence in men and women, whereas the mortality of colorectal cancer has a second position in women and third position in men.
Most colorectal cancers arise from adenomatous polyps. The risk of CRC increases with polyp size, number of polyps, and histology (eg, villous worse than tubular architecture). The recognition of larger size and more advanced histologic features as independent risk factors for the presence of invasive cancer within an adenoma has led to the use of the term ‘’advanced adenoma’’ for adenomas with a diameter of 1 cm or larger or have advanced histologic features (tubulovillous or villous histologic features of high-grade dysplasia). Older age is another risk factor for CRC. CRC is a rare diagnosis before the age of 40, the incidence begins to increase significantly between the ages of 40 and 50. The lifetime incidence for patients at average risk is 5 percent, with 90 percent of cases occurring after the age of 50.
Previous studies have shown a change in the distribution of CRC as well as the age of onset. Over the last 50 years, a gradual shift toward right sided colon cancers has been observed internationally. The greatest increase in incidence is in caecal primaries. Also the mean age of the CRC patient has increased in China and in Japan. On the other hand, studies from western countries show that the rates of colorectal cancer are increasing in young adults.
The study has implication in the future planning of screening strategies. Advanced polyps are detected at a younger age among men undergoing colonoscopy. Regarding the similar incidence of cancer in time, it is possible that colonoscopy is an effective way to prevent CRC in younger males by early intervention. Our data also indicates that sigmoidoscopy would be insufficient as a screening tool.
This is a well-written study investigating the prevalence of advanced polyps and associations with clinical characteristic in a large number of consecutive patients undergoing colonoscopy between 1998 and 2005. A major finding of the study was that the number of advanced polyps increased and this was associated with a shift towards younger age-groups and proximal location. This paper is a valuable addition to the literature on colorectal cancer screening and prevention.
S- Editor Zhu LH L- Editor Alpini GD E- Editor Liu Y
| 1. | Authority HKCRH. Fast stats for colorectum cancer 2003. Available from: http: //www3.ha.org.hk/cancereg/data/colorectum.pdf. |
| 2. | Axtell LM, Chiazze L. Changing relative frequency of cancers of the colon and rectum in the United States. Cancer. 1966;19:750-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Abrams JS, Reines HD. Increasing incidence of right-sided lesions in colorectal cancer. Am J Surg. 1979;137:522-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 53] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Kee F, Wilson RH, Gilliland R, Sloan JM, Rowlands BJ, Moorehead RJ. Changing site distribution of colorectal cancer. BMJ. 1992;305:158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Obrand DI, Gordon PH. Continued change in the distribution of colorectal carcinoma. Br J Surg. 1998;85:246-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Miller A, Gorska M, Bassett M. Proximal shift of colorectal cancer in the Australian Capital Territory over 20 years. Aust N Z J Med. 2000;30:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Cucino C, Buchner AM, Sonnenberg A. Continued rightward shift of colorectal cancer. Dis Colon Rectum. 2002;45:1035-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | O'Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH, Ko CY. Rates of colon and rectal cancers are increasing in young adults. Am Surg. 2003;69:866-872. [PubMed] |
| 9. | Li M, Gu J. Changing patterns of colorectal cancer in China over a period of 20 years. World J Gastroenterol. 2005;11:4685-4688. [PubMed] |
| 10. | Takada H, Ohsawa T, Iwamoto S, Yoshida R, Nakano M, Imada S, Yoshioka K, Okuno M, Masuya Y, Hasegawa K. Changing site distribution of colorectal cancer in Japan. Dis Colon Rectum. 2002;45:1249-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Eide TJ, Stalsberg H. Polyps of the large intestine in Northern Norway. Cancer. 1978;42:2839-2848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Jass JR. Do all colorectal carcinomas arise in preexisting adenomas? World J Surg. 1989;13:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Morson B. President's address. The polyp-cancer sequence in the large bowel. Proc R Soc Med. 1974;67:451-457. [PubMed] |
| 14. | Peipins LA, Sandler RS. Epidemiology of colorectal adenomas. Epidemiol Rev. 1994;16:273-297. [PubMed] |
| 15. | Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1379] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 16. | Kim EC, Lance P. Colorectal polyps and their relationship to cancer. Gastroenterol Clin North Am. 1997;26:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8087] [Cited by in RCA: 8005] [Article Influence: 228.7] [Reference Citation Analysis (1)] |
| 18. | Paraskeva C, Corfield AP, Harper S, Hague A, Audcent K, Williams AC. Colorectal carcinogenesis: sequential steps in the in vitro immortalization and transformation of human colonic epithelial cells (review). Anticancer Res. 1990;10:1189-1200. [PubMed] |
| 19. | Hoff G, Sauar J, Vatn MH, Larsen S, Langmark F, Moen IE, Foerster A, Thiis-Evensen E. Polypectomy of adenomas in the prevention of colorectal cancer: 10 years' follow-up of the Telemark Polyp Study I. A prospective, controlled population study. Scand J Gastroenterol. 1996;31:1006-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Sung JJ, Chan FK, Leung WK, Wu JC, Lau JY, Ching J, To KF, Lee YT, Luk YW, Kung NN. Screening for colorectal cancer in Chinese: comparison of fecal occult blood test, flexible sigmoidoscopy, and colonoscopy. Gastroenterology. 2003;124:608-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Cheng TI, Wong JM, Hong CF, Cheng SH, Cheng TJ, Shieh MJ, Lin YM, Tso CY, Huang AT. Colorectal cancer screening in asymptomaic adults: comparison of colonoscopy, sigmoidoscopy and fecal occult blood tests. J Formos Med Assoc. 2002;101:685-690. [PubMed] |
| 22. | Soon MS, Kozarek RA, Ayub K, Soon A, Lin TY, Lin OS. Screening colonoscopy in Chinese and Western patients: a comparative study. Am J Gastroenterol. 2005;100:2749-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Khan A, Shrier I, Gordon PH. The changed histologic paradigm of colorectal polyps. Surg Endosc. 2002;16:436-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Isbister WH. Colorectal polyps: an endoscopic experience. Aust N Z J Surg. 1986;56:717-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Smith D, Ballal M, Hodder R, Selvachandran SN, Cade D. The adenoma carcinoma sequence: an indoctrinated model for tumorigenesis, but is it always a clinical reality? Colorectal Dis. 2006;8:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Larsen IK, Grotmol T, Almendingen K, Hoff G. Lifestyle as a predictor for colonic neoplasia in asymptomatic individuals. BMC Gastroenterol. 2006;6:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Le Marchand L, Wilkens LR, Kolonel LN, Hankin JH, Lyu LC. Associations of sedentary lifestyle, obesity, smoking, alcohol use, and diabetes with the risk of colorectal cancer. Cancer Res. 1997;57:4787-4794. [PubMed] |
| 28. | Lieberman DA, Prindiville S, Weiss DG, Willett W. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290:2959-2967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 350] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 29. | Negri E, Braga C, La Vecchia C, Franceschi S, Filiberti R, Montella M, Falcini F, Conti E, Talamini R. Family history of cancer and risk of colorectal cancer in Italy. Br J Cancer. 1998;77:174-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Steinmetz KA, Potter JD. Food-group consumption and colon cancer in the Adelaide Case-Control Study. II. Meat, poultry, seafood, dairy foods and eggs. Int J Cancer. 1993;53:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |