INTRODUCTION
Liver ischemia/reperfusion (I/R) injury is a complication associated with hepatic resection, liver transplantation, and hemorrhagic shock with fluid resuscitation[1]. The consequences of I/R injury include liver and remote organ failure, both of which have high rates of morbidity and mortality. Despite improvements in organ protection and surgical techniques, I/R injury remains an important clinical problem, and there is significant interest in preventing this complication[2].
Hedgehog (Hh) proteins act as morphogens in several tissues during embryonic development[3-7]. In mammals, there are three such genes: Sonic hedgehog (Shh), Indian hedgehog (Ihh), and Desert hedgehog (Dhh)[8]. Sonic hedgehog (Shh) is the most extensively expressed gene during embryonic development[9,10], and lack of Shh in mice is lethal resulting in multiple defects, beginning in the early to mid gestational period[4-6]. Several new findings point to the involvement of Hh in inducing vascularization of several embryonic tissues. For example, hypervascularization of neuroectoderm is seen following transgenic over expression of Shh in the dorsal neural tube[11].
Inducible nitric oxide synthase (iNOS) from hepato-cytes and Kupffer cells is activated to produce nitric oxide (NO). Both cytotoxic and cytoprotective effects of NO have been demonstrated, related respectively, to its ability to restore microcirculation via its vasodilatory properties and to the inhibitory effect of large amounts of NO on energy metabolism, leading to cell death[12]. Recently, Pola et al[13] suggested that Shh is a potent angiogenic factor, and when administered to aged mice is able to induce robust neovascularization of ischemic hind-limbs. The aim of this study was to investigate the role of Shh on the course of liver I/R in rats and the interaction between treatment with nitric oxide donor L-Arginine-methyl ester (L-Arg) and up-regulation of Shh expression.
MATERIALS AND METHODS
Thirty male Sprague-Dawley rats weighing 220 to 240 g were used in the study. All the study protocols were performed according to the guidelines for the ethical treatment of experimental animals.
Animals and experimental protocol
The rats were housed individually in cages, were allowed free access to standard rat chow and water before and after the experiments. The animal rooms were windowless with temperature (22 ± 2°C) and lighting controls. The animals were fasted overnight before the experiments but were given free access to water. They were anesthetized with 100-mg/kg ketamine and 20-mg/kg xylazine. Right femoral vein was cannulated for administration of drugs and saline.
The animals were randomly separated into 3 groups, each containing 10 rats. Sham-control group (G1, n = 10): to determine the baseline values of histological parameters, we performed a sham operation. A midline incision was made in the upper abdomen and the liver was freed from its ligaments (except for liver I/R). I/R-untreated group (G2, n = 10): rats were subjected to the surgical procedure described below and underwent liver ischemia for 1 h followed by reperfusion for 45 min. Group 3 (I/R-L-Arg): after performing the same surgical procedure as in group 2, L-arginine-methyl ester (200 μg/kg per minute IV; Sigma Chemical Co, St. Louis, USA), an NO donor, was infused for 45 min by an infusion pump.
Liver ischemia-reperfusion
The ligament attachments connecting the liver to the diaphragm, abdominal wall, and neighboring organs were divided. After the organ had been carefully isolated, the liver hilum was exposed and the common hepatic artery and portal vein were identified. A vascular microclamp was used to interrupt the blood supply to three-quarters of the liver for 60 min, followed by 45 min of reperfusion. Other rats were subjected to a sham operation (sham-operated), namely an identical surgical procedure as in the I/R group was performed, but without vascular clamping; the rats were maintained under anesthesia for the duration of the experiment. At the end of the study protocol, the rats were sacrificed by an overdose of sodium pentobarbital.
Measurement of serum liver enzymes
The abdominal aorta was punctured and 5 mL blood was collected in heparinized tubes. Plasma was separated by centrifugation (3000 RPM for 10 min at room temperature) for biochemical studies. The levels of alanine aminotransferase (ALT, a specific marker for hepatic parenchymal injury) (units/L), aspartate aminotransferase (AST, a nonspecific marker for hepatic injury) (units/L), lactate dehydrogenase (LDH, a marker of nonspecific cellular injury) and γ-glutamyltranspeptidase (GGT, a sensitive but nonspecific indicator of liver disease with increased activity found in both cholestasis and hepatocellular damage) (units/L) in plasma were determined by standard auto-analyzer methods on an Abbot Aeroset (USA). The livers were obtained for histopathological evaluation just before the rats were sacrificed.
Malondialdehyde determination
Liver MDA levels were determined by Wasowiczs’s method (14) based on the reaction of MDA with thiobarbituric acid at 95-100°C. Fluorescence intensity was measured in the upper n-butanol phase by a fluorescence spectrophotometry, (Hitachi, Model F-4010) adjusted exitation at 525 nm and emission at 547 nm. Arbitrary values obtained were compared with a series of standard solutions (1, 1, 3, 3 tetramethoxypropane). Results were expressed as nanomole per gram wet tissue (nmol/g wet tissue).
Protein assays
The protein content of homogenates was determined according to the method of Lowry et al[15].
Histopathological study
The exteriorized livers were divided into two parts. One part was immediately placed in 10% formalin solution overnight and embedded in paraffin blocks. The blocks were sectioned 4-μm thick and stained with hematoxylin-eosin, according to standard protocols. Sections were evaluated by a point-counting method for severity of hepatic injury using an ordinal scale as follows: grade 0, minimal or no evidence of injury; grade 1, mild injury consisting of cytoplasmic vacuolation and focal nuclear pyknosis; grade 2, moderate to severe injury with extensive nuclear pyknosis, cytoplasmic hypereosinophilia, and loss of intercellular borders; and grade 3, severe necrosis with disintegration of hepatic cords, hemorrhage, and neutrophil infiltration.
Biochemical analyses
The second portion of the liver was washed in ice-cold 0.9% saline solution, weighed and stored at -70°C. Homogenates of the tissues were prepared as 1.0 g/10 mL in 250 mmol/L sucrose, 1 mmol/L EDTA, 1 mmol/L-dl-dithiothreitol and 15 mmol/L Tris HCl (pH 7.4), using an all-glass Potter Elvehjem homogenizer (Selecta, Barcelona, Spain). Each homogenate was centrifuged for 20 min at 800 ×g. The resulting supernatant fraction was used to determine enzyme activities. The protein concentrations of the supernatant were determined by the method described by Bradford[16].
Immunohistochemistry
The expressions of Shh in the liver tissue were analyzed by using an immunohistochemistry technique. Tissues were fixed in Bouin’s solution and embedded in paraffin. Sections were cut 4 μm in thickness, dewaxed in xylene, and incubated for 20 min in 0.3% H2O2 to block endogenous peroxidase activity. Sections were placed in a microwave for four minutes in phosphate buffer saline (PBS), and incubated with primary antibodies [monoclonal Anti-Sonic Hedgehog, N-terminal antibody produced in rat] overnight at room temperature. The primary antibody was visualized with diaminobenzidine (DAB) as a chromogen. The sections were counterstained in hematoxylene and eosin, cleared with xylene and covered with a cover slip. The tissue sections were examined, using a light microscope interfaced with a Zeiss Color Camera. The sections were analyzed by 2-3 observers who were blinded to the grouping of the animals, and categorized using a ranking system (0 = no immunoreactivity, 1 = faint, 2 = moderate, and 3 = strong immunoreactivity). At least 3 slides per animal were examined and the results were presented as average (= median).
Statistical analysis
Data was analyzed on an IBM compatible personal computer using SPSS version 9.0. All values were expressed as mean ± SE. The significance of the data obtained was evaluated by using analysis of variance (ANOVA). Differences between means were analyzed by using the post-ANOVA (Tukey’s b) test. P values of < 0.05 were considered significant.
RESULTS
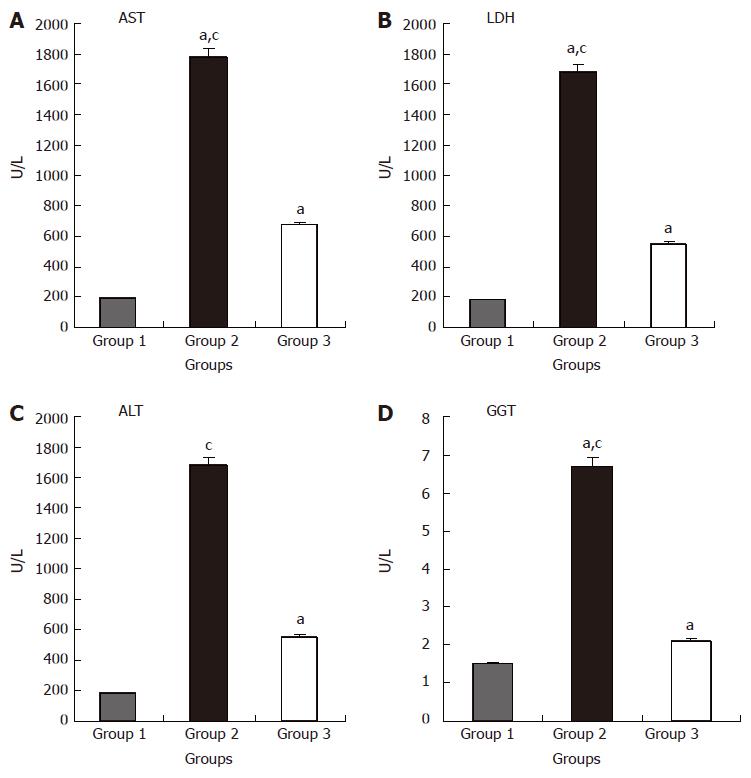
The biochemical measurements in the different study groups are shown in Figure 1 A-D. ALT, AST, GGT and LDH levels were significantly higher in groups 2 and 3 compared to group 1 (P < 0.05 for both comparisons). However, ALT, AST, GGT and LDH values were higher in group 2 compared to group 3 (P < 0.05).
Figure 1 Effects of L-Arg on liver function after liver I/R.
AST (A), LDH (B), ALT(C) and GGT (D) values in group 2 were significantly higher compared to groups 1 and 3 (aP < 0.05 vs group 1; cP < 0.05 vs group 3). Values are expressed as mean ± SE. GGT: γ-glutamyltranspeptidase; AST: aspartate aminotransferase; ALT: alanine aminotransferase. Group 1 (n = 10), Sham-control group; Group 2 (n = 10), I/R-untreated group; Group 3 (n = 10), I/R-L-Arg group.
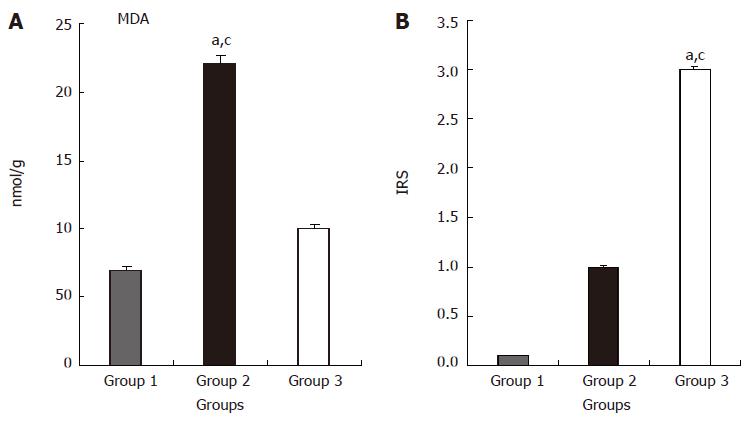
The values of MDA measurements in the different groups are shown in Figure 2A. MDA values were significantly higher in group 2 compared to groups 1 and 3 (P < 0.05 for both). In addition, MDA values were significantly higher in group 2 compared to group 3 (P < 0.05).
Figure 2 Effects of ischemia/reperfusion and L-Arg on MDA levels (A) and IRS (B) of liver tissue.
aP < 0.05 group 2 vs group 1; cP < 0.05 group 2 vs group 3. Values are expressed as mean ± SE, MDA; Malondialdehyde. IRS; Immunoreactivity scores, Group 1 (n = 10), Sham-control group; Group 2 (n = 10), I/R-untreated group; Group 3 (n = 10), I/R-L-Arg group.
The histopathological scores were 0.1 ± 0.2; 2.7 ± 0.6 and 1.3 ± 0.5 in groups 1, 2 and 3 respectively. The histopathological score was higher in groups 2 and 3 compared to group 1 (P < 0.05 for both). However, the histopathological score was significantly greater in group 2 compared to group 3 (P < 0.05).
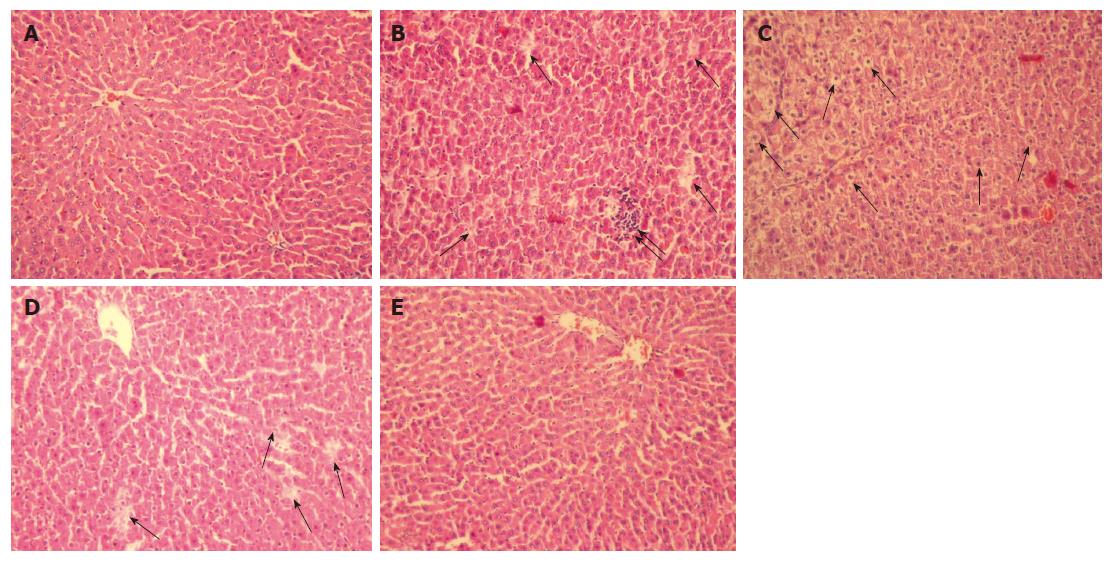
No morphological damage was observed in any of the rats in group 1 (Figure 3A). In group 2, the hepatocytes were markedly swollen, with striking vacuolization. In addition, areas of necrosis and cell infiltration were noted (Figure 3B-D). Group 3 showed well-preserved liver parenchyma with hepatocytes extending from the central vein. The regular sinusoidal structures showed normal morphology, without any signs of congestion (Figure 3E).
Figure 3 A: Group 1 shows normal liver parenchyma with regular morphology of both the hepatocytes and the sinusoids around the central vein (HE; x 200); B: Group 2 shows increase in necrosis (↑) and cell infiltration (↑↑); C: Swollen hepatocytes with marked vacuolization; D: necrosis (↑) (HE, x 200); E: Group 3 rats showing normal morphology of hepatocytes and sinusoids, reflecting well-preserved liver parenchyma (HE, x 200).
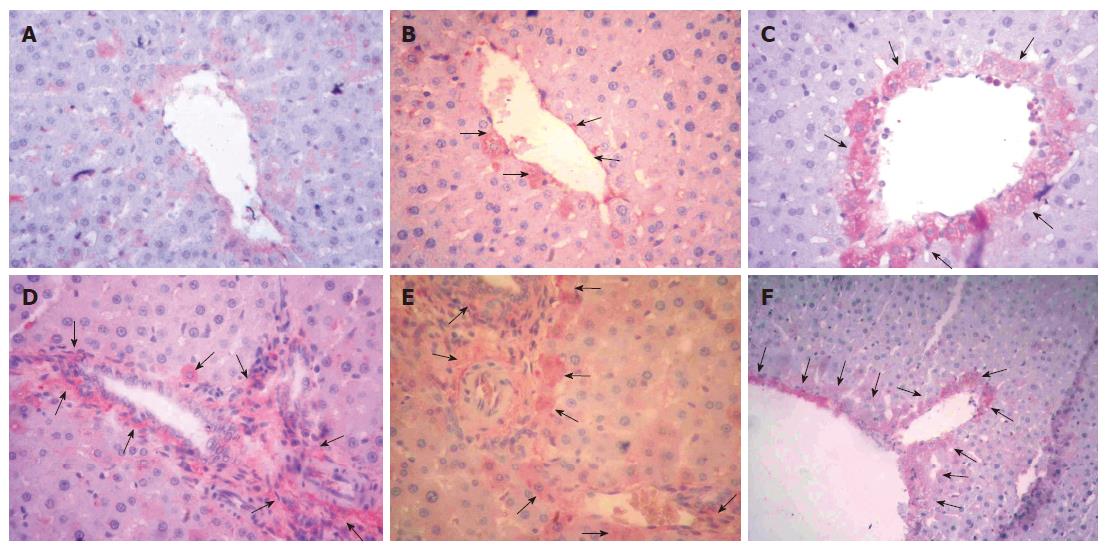
Immunoreactivity scores (IRS) in the three groups were 0.1 ± 0.1, 3 ± 0.1 and 1 ± 0.1 respectively. The IRS was significantly higher in the I/R-L-Arg group compared to the sham treated and I/R groups (P < 0.05 for both) (Figure 2B). There were no positive immunoreactive cells in the sham-operated rats (Figure 4A). Mild Shh positive immunostaining was detected in group 2 animals (Figure 4B). By contrast, the liver tissue from I/R-L-Arg rats showed intense expression of immunoreactive cells (Figure 4C-F).
Figure 4 A: No expression of Shh in the control group (Immunoperoxidase.
X 200); B: Mild Shh-positive immunostaining in group 2 (arrows) (Immunoperoxidase, X 200), C, D, E, F: Intense Shh expression in liver tissue from I/R-L-Arg treated rats (arrows) (Immunoperoxidase, X 200).
DISCUSSION
The Hedgehog family has been examined extensively during embryogenesis. Most of the prenatal studies have focused on the function of Hh family members in the regulation of epithelial-mesenchymal interactions that are crucial to the formation of limbs, lungs, gut, hair follicles, and bone[3-7], including a possible role in vascularization of certain embryonic tissues[17-19]. By contrast, the role for the Hh family in the regulation of adult tissue regeneration and revascularization has received little attention[20-22]. The intracellular signalling events mediated by Shh remain poorly defined. After synthesis, Shh undergoes autoproteolysis by an enzymatic activity contained within its carboxylterminal sequence, which leads to an aminoterminal product responsible for the biological activity of the protein, anchored to the cell membrane by a lipidic molecule[23,24]. It has been suggested that transduction of the Shh signal involves a complex of two membrane proteins, Patched (Ptc) and Smoothened (Smo), which display homology with members of transporters and G-protein-coupled receptors, respectively[25-27]. Recently, it has also been proposed that a Hedgehog (Hh)-binding protein (Hip), a putative transmembrane protein with epidermal growth factor-like domains, is a receptor for Hh peptides and might be implicated in modulating Hh signalling[28]. Pola et al[13] demonstrated that exogenous administration of Shh induces neovascularization in both corneal and ischemic hind limb models of angiogenesis. Neovascularization induced by Shh appears to be mediated by stromal cells producing a combination of potent angiogenic factors, including vascular endothelial growth factor (VEGF), and angiopoietin-1 and 2[13]. Shh seems to work as an indirect angiogenic agent and may activate neovascularization through Shh/patched-1 signaling, specifically in mesenchymal cells[13]. In another study, Pola et al[29] suggested that the Hh pathway may be postnatally recapitulated in response to skeletal muscle ischemia, and concluded that in adult mice, a strong up-regulation of Shh and Ptc1 occurs during regeneration of ischemic skeletal muscle. In our study, ALT, AST, GGT, LDH and MDA levels were significantly higher in the I/R-untreated group. In group 2, the hepatocytes were swollen and showed marked vacuolization. There were no positive immunoreactive cells in the sham-operated rats, while mild Shh positive immunostaining was detected in group 2 animals.
Therefore, supplementation of nitric oxide in the form of nitric oxide donors can be used to correct the nitric oxide deficiency encountered in many disease states. Nitric oxide, known as an endothelium-derived relaxing factor, is formed from the terminal guanidino nitrogen atom of L-arginine by NO synthase[30]. Nitric oxide binds to the haem moiety of guanylate cyclase and increases its activity by 400-fold, catalyzing the conversion of guanosine triphosphate to cyclic guanosine monophosphate (cGMP). Elevation of cGMP relaxes the smooth muscles in blood vessels including the genitourinary tract, inhibits platelet aggregation and adhesion, and blocks the adhesion of white cells to the blood vessel wall[31-33]. It has been suggested that cGMP can influence the inductive effects of Shh on neural tissue[34]. In the present study, the biochemical parameters were superior in the I/R-L-Arg group than in the I/R-untreated group. Histological examination of the liver revealed regular sinusoidal structures in group 3, as opposed to swollen cells with marked vacuolization seen in group 2. In addition, the expression of immunoreactive cells was increased markedly in liver tissue from I/R-L-Arg rats. These findings suggest that NO may play an important role in the immunohistochemical expression of Shh molecule.
Lipid peroxidation, mediated by free oxygen radicals, is believed to be an important cause of destruction and damage to cell membranes. Membrane peroxidation can lead to changes in membrane fluidity and permeability and also to enhanced rates of protein degradation, eventually resulting in cell lysis[35]. MDA levels have been extensively used as markers of lipid peroxidation and lipid peroxidation damage in tissues, including cells and body fluids in both clinical and experimental studies[36,37] . We also examined the effect of L-Arg on lipid peroxidation as measured by MDA levels. L-Arg reversed the increase in MDA levels to a considerable extent, thereby confirming its antioxidant role in I/R.
In conclusion, our findings suggest that the Shh molecules may be critical factors in the pathophysiology of inflammatory liver injury induced by I/R. NO may play an important role in the immunohistochemical expression of these molecules.
S- Editor Liu Y L- Editor Anand BS E- Editor Ma WH