Published online Jul 21, 2007. doi: 10.3748/wjg.v13.i27.3742
Revised: April 10, 2007
Accepted: April 18, 2007
Published online: July 21, 2007
AIM: To investigate whether anti-H pylori antibodies have cross-reaction with antigens of erythrocyte membrane.
METHODS: Blood samples were collected from 14 volunteers (8 positive and 6 negative for H pylori detected by 13C-urea breath test) of the general population. Erythrocyte membrane proteins of the subjects were examined by Western blot using anti-H pylori serum. The proteins related to the positive bands were identified by mass spectrum analysis.
RESULTS: Anti-H pylori antibodies had cross-reaction with the proteins of about 50 kDa of erythrocyte membranes in all samples independent of H pylori infection. One protein in the positive band was identified as Chain S, the crystal structure of the cytoplasmic domain of human erythrocyte Band-3 protein.
CONCLUSION: Anti-H pylori antibodies cross-react with some antigens of human erythrocyte membrane, which may provide a clue for the relationship between H pylori infection and vascular disorders.
-
Citation: Guo FH, Yan XM, Fan CX, Zhao F, Hu Y, Xiao D, Zeng X, Zhang MJ, He LH, Meng FL, Zhang JZ. Cross-reactivity of anti-
H pylori antibodies with membrane antigens of human erythrocytes. World J Gastroenterol 2007; 13(27): 3742-3746 - URL: https://www.wjgnet.com/1007-9327/full/v13/i27/3742.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i27.3742
H pylori, first isolated by Marshall and Warren[1], a gram-negative spiral bacterium, colonizing in gastric mucosa, is notorious for causing chronic infections and has been linked to various gastric diseases such as chronic gastritis, peptic ulcer, gastric mucosa-associated lymphoid tissue lymphoma and gastric cancer[2-4]. In recent years, infection by H pylori has been linked to extradigestive pathologies including ischemic cardiac and cerebral diseases. Many seroepidemiological studies revealed the relationship between H pylori and vascular disorders[5,6] even though the prevalence of positive findings varied widely between studies and not all studies reported positive results[7-9]. However, the exact nature of the association is not completely elucidated.
Several investigations revealed that heat shock proteins (HSPs) of H pylori are extremely homologous with HSPs of humans[10], the O-side chain of the lipopolysaccharide (LPS) of a number of H pylori strains is structurally similar to the Lewis histo-blood group antigens[11], anti-CagA antibodies cross-reacted with antigens of blood vessels[12]. All these imply that autoimmunity might take part in pathomechanisms of H pylori.
The changes of erythrocytes affect the whole blood viscosity, which contributes importantly to thrombosis and atherosclerosis (AS). Our previous studies found that anti-H pylori serum reacted with parts of erythrocytes and endothelial cells of heart valves using immunohistochemical method[13,14]. But it remains unknown which antigen resulted in these positive reactions. The present study was aimed to investigate whether the proteins of erythrocyte membrane cross-react with anti-H pylori by Western blot assay and to identify the special proteins by mass-spectrum assay in an effort to provide a clue for pathogenic link between H pylori infection and vascular disorders.
Fresh blood samples were collected from 14 subjects from the general population whose results of 13C-urea breath test (13C-UBT) were supplied by Chinese People’s Liberation Army General Hospital. The kit for 13C-UBT was provided by Altachem Pharma Ltd. Current infection of H pylori was confirmed by a value of 13C-UBT greater than 4. General data about the subjects are shown in Table 1. Informed consents were obtained from all the volunteers before 13C-UBT and blood sampling.
| Subject No. | ||||||||||||||
| 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 | 12 | 13 | 14 | |
| Gender | F | F | M | M | F | M | M | F | F | M | M | M | F | F |
| Age (yr) | 27 | 45 | 44 | 44 | 31 | 49 | 32 | 42 | 47 | 42 | 34 | 45 | 28 | 30 |
| 13C-UBT (DOB) | 40.93 | - | 8.48 | - | - | 8.81 | 11.23 | - | 10.33 | 23.08 | - | - | 25.10 | 38.71 |
Fresh blood collected from the subjects were mixed with heparin as anti-coagulant. The erythrocytes were separated by centrifugation at 1230 ×g and were lysed with deionized water and then centrifuged at 12 000 ×g for 20 min at 4°C. The pellets were washed in three volumes of cold phosphate buffer at 5 mmol/L, pH 8.0, containing 1 mmol/L EDTA and 1 mmol/L PMSF (Sigma) 6 times until the membranes were white and then were resuspended in the same buffer and centrifuged at 30 000 ×g for 1 h at 4°C. The pellets were frozen at -80°C and dried at -56°C in cold vacuum. The membranes were resuspended in the 2-DE lysis buffer cocktail consisting of 7 mol/L urea, 2 mol/L thiourea, 10 g/L DTT, and 40 g/L CHAPS at 4°C for 2 h, then ultrasonicated on ice. The concentration of proteins in each sample was 6-12 g/L determined by Bradford protein assay[15]. The whole proteins of H pylori NCTC11637 were extracted as positive. All reagents in 2-DE lysis buffer were bought from Amersham.
SDS-PAGE was performed using a Bio-Rad Mini-Protean 3 electrophoresis cell. Approximately 120 μg of membrane proteins were parallelly loaded into two wells of 10% SDS-polyacrylamide minigel, 60 μg per well. Thirty μg of whole proteins of H pylori NCTC11637 as positive control and 5 μL prestained molecular weight standards marker (Fermantas) were also respectively loaded in two wells per gel.
Proteins were transferred to a PVDF membrane (Amersham) using Bio-Rad Semi-Dry transfer unit. Blocking was performed overnight at 4°C in blocking buffer (TBS containing 50 g/L BSA). The membrane was bisected and one part was incubated with the primary antibody, rabbit anti-H pylori NCTC11637 serum (from immunized rabbits with H pylori NCTC11637, the animals were provided by Vital River Laboratories Co. Ltd. and raised by the Department of Laboratory Animal Science, Peking University Health Science Center) for 2 h at room temperate (RT). To exclude the color reaction resulting from the direct conjugation of the second antibody and the normal serum with the proteins on PVDF membranes, the normal serum (pre-immunization serum) of the same rabbits was used as control for another part of membranes with the same samples. Other steps were performed according to the Western blot assay. The second antibody, goat anti-rabbit IgG AP conjugate and AP substrates were from Vector.
The blots incubated in anti-H pylori serum were compared with the others of the same sample incubated in normal serum to find out the different reacted bands. The samples were chosen according to different bands and SDS-PAGE was performed and the gel was stained with Coomassie blue-R250 dye. The bands in the SDS-PAGE gel in accordance with different reacted ones in Western blot were excised, and in-gel reduction, alkylation and trypsin digestion was performed according to EMBL protocol (http://www.proteomics.com.cn/paper/InGel.html). Briefly, after a washing step, gel particles were reduced with DTT and alkylated with iodoacetamide. A second washing was performed before overnight digestion with 3 μL (40 mg/L) trypsin (Sigma). The resulting peptides were extracted with 500 mL/L ACN and 50 mL/L TFA and dried in a cold vacuum.
The digested samples were mixed with a saturated matrix solution (1:1) (α-cyano-4-hydroxycinnamic acid prepared in 500 mL/L acetonitrile and 1 mL/L formic acid). All mass spectra were obtained on a 4700 Proteomics analyzer with TOF/TOF optics (Applied Biosystems, Foster City, CA, USA) in the positive ion reflector mode with a mass accuracy of about 50 ppm. The MALDI tandem mass spectrometer used a 200 Hz frequency-tripled Nd:YAG laser operating at a wavelength of 355 nm. MS spectra were obtained between Mr 800 and 4000 with ca. 1000 laser shots. MS/MS spectra were acquired with 2000 laser shots using air as the collision gas. The singly charged peaks were analyzed using an interpretation method present in instrument software, where the five most intense peaks were selected and MS/MS spectra were generated automatically, excluding those from the matrix, due to trypsin autolysis peaks. Spectra were processed and analyzed by the Global Protein Server Workstation (Applied Biosystems, Foster City, CA, USA), which uses internal Mascot v2.0 software(Matrix Science, UK) for searching the peptide mass fingerprints and MS/MS data. Searches were performed against the NCBI non-redundant protein database (updated 18 November 2005). Identifications with a GPS confidence interval of greater than 95% were accepted.
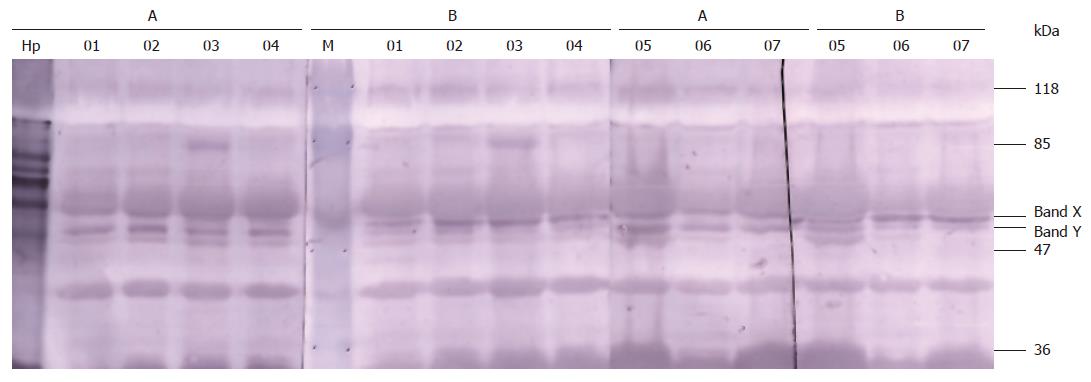
Both normal rabbit serum and anti-H pylori serum showed immunoreactivities with the membrane proteins of about 110 kDa, 55 kDa, 51kDa, 50 kDa, 40 kDa and 27 kDa of all erythrocytes. However, anti-H pylori serum specially recognized antigens of about 50 kDa (marked as band Y in Figure 1) from erythrocytes compared with the normal serum. Remarkably, this feature existed not only in H pylori+ subjects (No. 01, 03, 06, 07, 09, 10, 13, 14) but also in H pylori - subjects (No. 02, 04, 05, 08, 11, 12). The immunoreactivity of another band (marked as band X in Figure 1) with anti-H pylori serum was weaker than that with normal serum.
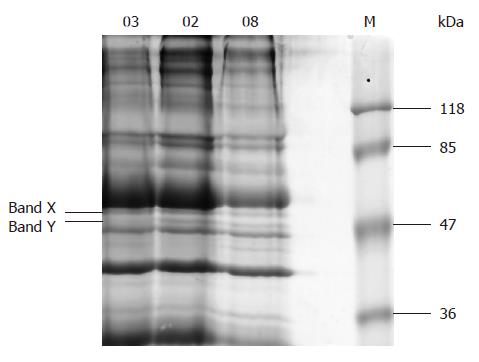
There were 17-18 bands in the SDS-PAGE 10% gel of erythrocyte membrane protein sample (Figure 2). The special band of about 50 kDa and another one closely above it (respectively marked as band Y and band X in Figure 2) corresponding to the specially reacted bands in Western blot were faintly stained. Five proteins were identified in the two bands, 4 in band X and 1 in band Y (Table 2).
| Band code | Protein name (source) | Accession No. | Protein score | Protein acore CI% | Protein MW | Protein PI |
| X | Flotillin 1 (Sus scrofa) | gi|41529176 | 88 | 99.560 | 47 325.6 | 7.66 |
| Flotillin 1 (Macaca mulatta) | gi|55700801 | 87 | 99.433 | 47 383.6 | 6.71 | |
| Flotillin 1 variant (Homo sapiens) | gi|62896619 | 87 | 99.378 | 47 324.6 | 8.18 | |
| Predicted: similar to flotillin-1 isoform 5 (Canis familiaris) | gi|73972134 | 84 | 98.640 | 34 070.7 | 8.52 | |
| Y | Chain S, crystal structure of the cytoplasmic domain | gi|14277742 | 93 | 99.861 | 42 509.3 | 4.49 |
| of human erythrocyte Band-3 protein (Homo sapiens) |
The pathogenesis of ischemic vascular diseases is multifactorial. AS and thrombosis, the principle basis of ischemic vascular disease, determine the occurrence of ischemic events. However, many AS patients lack traditional risk factors, suggesting that other mechanisms may be involved in the AS development[16,17]. In recent years, more attention has been paid to the relationship between infection and ischemic diseases[16,18,19].
Several studies indicated the association between H pylori infection and ischemic vascular disease especially when the CagA+ strain was involved[5,6], although the results are currently being debated[7-9]. By now, most studies have been based on seroepidemiology and nonspecific systemic inflammation. The exact mechanisms by which H pylori infection contributes to the progression of vascular disorders have not been elucidated.
The molecular mimicry between elements of H pylori and those of host cells[10,11] provides clues for autoimmunity as one of the candidate pathopoiesis. Franceschi and his colleagues[12] reported that anti-CagA antibodies cross-reacted with antigens of both normal and atherosclerotic blood vessels by immunohistochemistry and anti-CagA antibodies also specifically immunoprecipitated two antigens of 160 and 180 kDa from both normal and atherosclerotic artery lysates. The authors speculated that the immunoprecipitated proteins were not CagA of H pylori but vascular elements because the two antigens were different from CagA (about 116-140 kDa) in molecular weight. The reactivity detected in vessels with anti-CagA antibodies was caused by the mimicking vascular antigens. We think this speculation reasonable. However, the two antigens were not identified. Moreover, the difficulty in obtaining vascular tissue makes the investigation in the relationship between vascular endothelium and H pylori infection unfruitful.
Erythrocyte is one of most important factors affecting hemodynamics. Its membranes can be easily isolated in large quantities and many blood group antigens are expressed not only on the surface of blood cells but also on vascular endothelial cells. Thus, we chose erythrocyte to investigate the cross-reaction of human plasma membrane and anti-H pylori antibodies. Our previous study showed that anti-H pylori serum reacted with erythrocytes by immunohistochemical method[13]. But we did not know which elements resulted in the immunoreaction and whether the elements belong to erythrocytes or to H pylori. In the present investigation, antigens of about 50 kDa from erythrocyte membrane strongly immunoreacted with anti-H pylori serum rather than normal serum in all 14 samples (Figure 1). This feature did not depend on current infection of H pylori. Therefore, we speculate the reacted antigens are not elements of H pylori but the mimicking erythrocyte antigens. The results of mass spectrum assay confirmed our speculation. One protein was identified as Chain S, the crystal structure of the cytoplasmic domain of human erythrocyte Band-3 protein (Mr 42.5 kDa) in the special band (band Y in Figure 2).
Band 3 protein is the most abundant transmembrane protein to maintain the normal metabolism and function of human erythrocyte. This protein of about 95-100 kDa has two domains. The N-terminal domain of about 40 kDa is located within the cytoplasm and participates in signal transmission across membranes and other functions such as growth, differentiation and interaction of cellules, while the C-terminal of 55 kDa domain is membrane-associated and mediates the exchange transportation of anions Cl-/ HCO3- across the erythrocyte membrane[20,21]. In this study, the two antigens of 160 and 180 kDa mimicking with CagA were not found possibly because of the diversity of erythrocytes and vascular cells.
We consider that antibodies against H pylori may not contact with cytoplasmic domain of Band 3 of normal erythrocyte. However, oxygen free radicals and systemic inflammation caused by acute or chronic infection could damage erythrocyte membrane leading to the decrease of erythrocyte deformability, increase of erythrocyte fragility and elevation of erythrocyte aggregation index. Some authors reported these changes in several ischemic cardiac disease patients with H pylori infection[22]. The impaired erythrocytes might be easier to be disrupted, inducing internal antigens (including the cytoplasmic domain of Band 3 protein) to be exposed to circulating antibodies. Then anti-H pylori antibodies could bind the exposed antigens and cause inflammatory cell activation, which might be associated with the changes of hemorheology and hemodynamics, plaque aggregation, thrombus formation and atherogenesis leading to ischemic events.
In band X (Figure 2), 4 proteins were identified, which were considered to be flotillin 1 variants according to their resource and molecular weight. The reason why the reaction of the band X incubated with normal serum was stronger than with anti-H pylori serum is being investigated.
The protein that cross-reacted with anti-H pylori antibodies probably is another one that we could not identify due to its trace quantity and the limit of separation ability of SDS-PAGE. Nevertheless, our study provides an experimental evidence of molecular mimicry between H pylori antigens and erythrocyte membrane proteins. The results support the hypothesis that autoimmunity induced by H pylori infection plays an important role not only in vascular disorders but also in various extragastric diseases.
The pathogenesis of ischemic vascular diseases is multifactorial. The conventional risk factors do not fully account for the risk of these diseases. In recent years, more attention has been paid to the relationship between infection and ischemic diseases. Several studies indicated the association between H pylori infection and vascular disorders. However, the exact nature of the association is not completely elucidated.
The molecular mimicry between elements of H pylori and those of host cells provides clues for autoimmunity as one of candidate pathopoiesis. Autoimmunity has become one of the hot spots of studies in recent years. Some studies have found that anti-H pylori antibodies reacted with endothelial cells and erythrocytes.
This study choose erythrocyte, which is easily to be isolated in large quantities, to investigate the cross-reaction of human plasma membrane and anti-H pylori antibodies and found anti-H pylori antibodies cross-reacted with the proteins of about 50 kDa of erythrocyte membranes in Western blot. The protein was identified by mass spectroscopy.
Erythrocyte is one of most important factors affecting hemodynamics. Many blood group antigens are expressed not only on the surface of blood cells but also on vascular endothelial cells. The materials selecting and the results of this study provide a new clue and experimental evidence for autoimmunity as one of the potential pathopoiesis of H pylori infection in vascular disorders.
This study looks at the cross-reaction of human plasma membrane and anti-H pylori antibodies. Although the contribution of the cross-reaction to the relationship between H pylori infection and vascular disorders is not clear, this study provides some interesting observations and a new clue for autoimmunity as one of the potential pathopoiesis of H pylori infection.
S- Editor Liu Y L- Editor Ma JY E- Editor Ma WH
| 1. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3302] [Cited by in RCA: 3265] [Article Influence: 79.6] [Reference Citation Analysis (1)] |
| 2. | Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 612] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 3. | Hobsley M, Tovey FI, Holton J. Precise role of H pylori in duodenal ulceration. World J Gastroenterol. 2006;12:6413-6419. [PubMed] |
| 4. | Chen LT, Lin JT, Tai JJ, Chen GH, Yeh HZ, Yang SS, Wang HP, Kuo SH, Sheu BS, Jan CM. Long-term results of anti-Helicobacter pylori therapy in early-stage gastric high-grade transformed MALT lymphoma. J Natl Cancer Inst. 2005;97:1345-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Pietroiusti A, Diomedi M, Silvestrini M, Cupini LM, Luzzi I, Gomez-Miguel MJ, Bergamaschi A, Magrini A, Carrabs T, Vellini M. Cytotoxin-associated gene-A--positive Helicobacter pylori strains are associated with atherosclerotic stroke. Circulation. 2002;106:580-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | de Luis DA, Lahera M, Cantón R, Boixeda D, San Román AL, Aller R, de La Calle H. Association of Helicobacter pylori infection with cardiovascular and cerebrovascular disease in diabetic patients. Diabetes Care. 1998;21:1129-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Khairy P, Rinfret S, Tardif JC, Marchand R, Shapiro S, Brophy J, Dupuis J. Absence of association between infectious agents and endothelial function in healthy young men. Circulation. 2003;107:1966-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Koenig W, Rothenbacher D, Hoffmeister A, Miller M, Bode G, Adler G, Hombach V, März W, Pepys MB, Brenner H. Infection with Helicobacter pylori is not a major independent risk factor for stable coronary heart disease: lack of a role of cytotoxin-associated protein A-positive strains and absence of a systemic inflammatory response. Circulation. 1999;100:2326-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Haider AW, Wilson PW, Larson MG, Evans JC, Michelson EL, Wolf PA, O'Donnell CJ, Levy D. The association of seropositivity to Helicobacter pylori, Chlamydia pneumoniae, and cytomegalovirus with risk of cardiovascular disease: a prospective study. J Am Coll Cardiol. 2002;40:1408-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Lamb DJ, El-Sankary W, Ferns GA. Molecular mimicry in atherosclerosis: a role for heat shock proteins in immunisation. Atherosclerosis. 2003;167:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Appelmelk BJ, Simoons-Smit I, Negrini R, Moran AP, Aspinall GO, Forte JG, De Vries T, Quan H, Verboom T, Maaskant JJ. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect Immun. 1996;64:2031-2040. [PubMed] |
| 12. | Franceschi F, Sepulveda AR, Gasbarrini A, Pola P, Silveri NG, Gasbarrini G, Graham DY, Genta RM. Cross-reactivity of anti-CagA antibodies with vascular wall antigens: possible pathogenic link between Helicobacter pylori infection and atherosclerosis. Circulation. 2002;106:430-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 13. | Sun SF, Xiao WP, Zhao L, Zhang JZ, Liu RC, He LH, Feng TQ, Zhao S. Immunohistochemical analysis of H pylori associated antigens on the surface of red blood cells. Zhonghua Neike Zazhi. 2000;39:764. |
| 14. | Xiao WP, Sun SF, Zhang JZ, Liu RC, Feng TQ, Yu PL, Zhao L, Zhao S, He LH. Detection of H pylori associated antigens in vascular tissue of patients with rheumatic heart disease. Zhonghua Weishengwuxue He Mianyixue Zazhi. 2000;20:430. |
| 15. | Steel LF, Mattu TS, Mehta A, Hebestreit H, Dwek R, Evans AA, London WT, Block T. A proteomic approach for the discovery of early detection markers of hepatocellular carcinoma. Dis Markers. 2001;17:179-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Wang JL. Microbial infection, inflammtion and atherosclerosis. Zhongguo Shiyan Zhenduanxue. 2003;7:3-7. |
| 17. | Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke. 2003;34:2518-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 18. | Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II2-I10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 542] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 19. | Grau AJ, Buggle F, Heindl S, Steichen-Wiehn C, Banerjee T, Maiwald M, Rohlfs M, Suhr H, Fiehn W, Becher H. Recent infection as a risk factor for cerebrovascular ischemia. Stroke. 1995;26:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 135] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Tanner MJ, Martin PG, High S. The complete amino acid sequence of the human erythrocyte membrane anion-transport protein deduced from the cDNA sequence. Biochem J. 1988;256:703-712. [PubMed] |
| 21. | Zhang D, Kiyatkin A, Bolin JT, Low PS. Crystallographic structure and functional interpretation of the cytoplasmic domain of erythrocyte membrane band 3. Blood. 2000;96:2925-2933. [PubMed] |
| 22. | Liu DN, He ZY, Li JS. Relationship between Helicobacter pylori infection, virulence of Hp and myocardial infarction. Zhongguo Weixunhuan Zazhi. 2004;8:26-29. |