Published online Jul 21, 2007. doi: 10.3748/wjg.v13.i27.3684
Revised: April 23, 2007
Accepted: April 26, 2007
Published online: July 21, 2007
AIM: To evaluate the potential of Polysol, a newly developed preservation solution, in cold storage of small bowel grafts, compared with the current standards, University of Wisconsin solution (UW), Celsior and histidine-tryptophan-ketoglutarate solution (HTK).
METHODS: Male Wistar rats were used as donors. Small bowels were retrieved, flushed and then stored in the respective 4 solutions for 18 h at 4°C. Functional integrity of the grafts was evaluated by isolated reperfusion with oxygenated Krebs-Henseleit buffer at 37°C for 30 min in all 4 groups.
RESULTS: Polysol preservation exhibited the highest tissue ATP concentration and the lowest release of LDH. Malondialdehyde, an index for tissue lipid peroxidation, was also the lowest in Polysol. Tissue oxygen consumption was significantly higher in Polysol than in the others. Of interest, UW-storage promoted 10-fold higher apoptosis than in the others. Moreover, electron microscopy revealed that the mucosal villi/micro-villi formation and the cell organelles, including mitochondria, were both significantly better preserved in Polysol, while deleterious alterations were apparent in the others, most notably in UW. Although Celsior and HTK exhibited the better trend of results than UW in some parameters, but could not reach the over-all superiority to UW.
CONCLUSION: Cold storage using Polysol resulted in significantly better integrity and function of small bowel grafts than UW. Hence, Polysol may be a novel alternative for the small bowel preservation.
- Citation: Wei L, Hata K, Doorschodt BM, Büttner R, Minor T, Tolba RH. Experimental small bowel preservation using Polysol: A new alternative to University of Wisconsin solution, Celsior and histidine-tryptophan-ketoglutarate solution? World J Gastroenterol 2007; 13(27): 3684-3691
- URL: https://www.wjgnet.com/1007-9327/full/v13/i27/3684.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i27.3684
In the last decade, small bowel transplantation (SBTx) has emerged as a life-saving option for patients with intestinal failure and life threatening complications of total parenteral nutrition. Although the recent advances in immunosuppressive agents or regimens, such as Tacrolimus or anti-CD25 antibody, have brought a “break though” in organ transplantation, the patient outcome after SBTx still remains inferior to other organ transplantations, such as heart, kidney or liver[1].
Of note, the small bowel is the most perfused organ under physiological conditions, receiving up to 25% of all cardiac output, most of which (up to 90%) is consumed in the mucosa and the submucosa, to sustain its large surface area (up to 100 m2) and its high turn over rate. This physiological feature of the gut leads, in turn, to the extreme vulnerability of the mucosal layer to ischemia during cold storage (CS). Although intestinal mucosa has high regenerative ability, Takeyoshi et al[2] demonstrated that morphological recovery of the injured ileal mucosa after 24 h CS requires at least one month. This prolonged damage from ischemia/reperfusion injury (IRI) surely participates not only in acute graft rejection but also in various postoperative complications, such as bacterial translocation (BT), endotoxin absorption and long-lasting malnutrition of the recipient. Moreover, there have been lines of evidence demonstrating that the incidence of BT was not different between allogenic and isogenic transplantation, in the latter no immunological rejections occurred[3,4]. These facts clearly suggest that non-immunological factors, such as IRI, seem to play an important role in the development of BT. Taken all these together, how to protect the mucosal integrity during organ preservation still remains of primary interest to further improve the clinical outcome of SBTx[5].
Meanwhile, the current clinical standard for small bowel preservation is cold static storage using the University of Wisconsin solution (UW). Despite its overall acceptance as the first choice for solid organs, UW is unable to effectively preserve the small bowel grafts[6,7]. Small bowel preservation using UW is rather disappointing since the storage time is restricted only to 6-8 h, thus limiting the area of organ delivery. This limitation, at least in part, interferes to select the most proper recipient of HLA matching, even though more suitable candidates are out of the transportable area. Moreover, with such a relatively short storage duration, the 1-year graft survival after SBTx is still 65%[8]. Other commercially-available preservation solutions, such as Histidine-Tryptophane-Ketoglutarate (HTK) and Celsior have not been used in clinical SBTx so far, mainly due to the lack of relevant evidence for their usefulness[7,9,10]. Accordingly, there has clearly been an urgent need for developing a novel refinement in small bowel preservation as well as for a reliable comparison study of standard preservation solutions for successful SBTx.
Recently, a new preservation solution, Polysol, was developed for hypothermic machine-perfusion preservation. Polysol is a lower viscosity solution than UW, while keeping a high oncotic pressure. Furthermore, Polysol contains free radical scavengers, various kinds of amino acids, vitamins and nutrients, all of which certainly counteract the adverse effects during CS. In fact, we have recently demonstrated, using a rat model of steatotic liver preservation, that Polysol exhibited a superior quality of such “marginal” organ preservation in CS[11]. These characteristic features of Polysol have led us to hypothesize that this solution might reduce the high susceptibility of the small bowel grafts to IRI, even in simple CS.
The present study was thus designed to investigate the potential of Polysol on preserving the integrity and the function of small bowel grafts in CS, and to compare its efficacy and feasibility with the current standards, UW, Celsior and HTK.
All animal experiments were performed in accordance with the federal German law regarding the protection of animals. This study also complied with institution guidelines as well as the criteria in “Guide for the Care and Use of Laboratory Animals” (NIH publication 86-23, revised 1985).
Male Wistar rats weighing between 250 g and 300 g were used as donors, and randomly assigned into four groups; UW, Celsior, HTK or Polysol group (n = 7 each). They all received humane care and had an acclimatization period of at least 1 wk under specific pathogen free conditions according to the FELASA (Federation of European Laboratory Animal Science Associations) recommendations.
UW (Via Span) was purchased from DuPont Pharma (Bad Homburg, Germany).
The HTK solution (Custodiol), from Dr. Franz Köhler Chemie GmbH (Alsbach-Hähnlein, Germany), Celsior from Genzyme (Neu-Isenburg, Germany), and Polysol from Doorzand Medical Innovations (Amsterdam, The Netherlands) were all provided free of charge for research purpose. The main components in the respective solutions are summarized in Table 1.
| Components | UW | Celsior | HTK | Polysol |
| Colloid (g/L) | HES (250 kDa), 50 | - | - | PEG (35 kDa), 20 |
| Na/K ratio (mmol/L) | 27/125 | 100/15 | 10/15 | 135/5 |
| Buffers (mmol/L) | H2PO4-, 25 | Histidine, 30 | Histidine, 180 | Histidine, 6.3 |
| H2PO4-, 21.74 | ||||
| HEPES, 20 | ||||
| Antioxidants (mmol/L) | Allopurinol, 1 | Glutathion, 3 | - | Allopurinol, 1.2 |
| Glutathion, 3 | Glutathion, 3 | |||
| Alpha-tocopherol, 5 × 10-5 | ||||
| Ascorbic acid, 0.11 | ||||
| Nutrients (mmol/L) | - | - | Glucose, 28 | Glucose, 11.1 |
| Adenine, 5 | ||||
| Sodium pyruvate, 0.23 | ||||
| Impermeants (mmol/L) | Lactobionate, 100 | Lactobionate, 80 | Raffinose, 3 | |
| Raffinose, 30 | Trehalose, 5.3 | |||
| Na+-Gluconate, 74.99 | ||||
| K+-Gluconate, 20 | ||||
| Amino acids (mmol/L) | - | Glutamic acid, 20 | Tryptophan, 2 | various1, 11 |
| Vitamins (mmol/L) | - | - | - | various2, 0.17 |
| Ca2+/Mg2+ (mmol/L) | - | 0.25/13 | 0.015/4 | 2/4 |
| Others (mmol/L) | Adenosine, 5 | Mannitol, 60 | Mannitol, 30 Ketoglutarate, 1 | Adenosine, 5 |
| pH | 7.4 | 7.3 | 7.2 | 7.4 |
| Osmolarity (mOsm/L) | 320 | 320 | 310 | 320 |
| Viscosity at 5°C (cP) | 5.7 | 1.3 | 1.8 | 1.8 |
Prior to the surgical procedures, rats were fasted for 24 h while having free access to water, and then anesthetized by intramuscular injection of ketamine (90 mg/kg) and xylazine (10 mg/kg). The abdomen was opened by a midline incision with bilateral subcostal extensions, and the total jejunum and ileum was isolated with the vascular pedicle, as described in detail elsewhere[12]. The rats were administered heparin (1000 U/kg) before the organ retrieval. The superior mesenteric artery (SMA) was cannulated with a 20-gauge polyethylene catheter and the small bowel was immediately flushed with 10 mL of the respective solutions at 4°C. The portal vein was cannulated with a polyethylene catheter (I.D. 1.5 mm), later connected to a silicone tube to allow the collection of the venous effluent upon reperfusion. A short 14-gauge cannula was inserted into the upper jejunal lumen and secured with a circumferential tie. The intestinal lumen was first flushed with 20 mL cold normal saline solution, followed by a rinse with 15 mL of the respective preservation solutions.
The excised small bowel grafts were stored ischemically for 18 h in a cold bath of the respective solutions (100 mL). The temperature was kept at 4°C constantly by an external cooling circuit (Ministat 125, Peter Huber Kältemaschinenbau GmbH, Germany).
The grafts were thereafter reperfused in vitro in a recirculating fashion at a constant flow rate of 8 mL/min for 30 min, according to the previously established techniques[12]. The grafts were weighed, and then gently reflushed with 5 mL of normal saline at 22°C via SMA. They were then transferred into a temperature-controlled (37°C) organ bath, filled with the modified Krebs-Henseleit buffer (KHB). The compositions were as follows: 50 g/L dextran 78, 9.5 g/L KHB, 0.37 g/L calcium chloride, 0.06 mg/L dexamethasone, 0.07 g/L atropine, and 0.21% sodium bicarbonate. The small bowel graft was floated, but almost completely immersed in KHB at 37°C, allowing homogeneous perfusion flow and temperature. The care was taken to avoid unnatural bending or distortion of the graft. Carbogen (95% O2 + 5% CO2) was used for oxygenation, and perfusate-pO2 was continuously kept over 500 mmHg during the whole reperfusion period. The venous effluent was collected intermittently through the portal vein catheter for biochemical analysis.
Adenosine triphosphate (ATP) concentration: At the end of reperfusion, tissue samples in all groups were snap frozen in liquid nitrogen and preserved below -80°C until later analysis. The samples were first cut into small pieces in liquid nitrogen and weighed (wet-weight), then freeze-dried in a high vacuum system at -40°C for six days (Beta 1-16, Martin Christ Gefriertrocknungs-Anlagen GmbH, Osterode, Germany). After tissue water was evaporated, the samples were weighed again (dry-weight). The tissue concentrations of ATP were determined by standard enzymatic tests, as described in detail elsewhere[13]. The results were ultimately corrected with the respective wet-weight per dry-weight ratio of the samples, and expressed in micromoles per gram of dry-weight.
Lactate dehydrogenase (LDH) release: Effluent activities of LDH were determined at the end of reperfusion using a commercially-available photometric kit (Boehringer, Mannheim, Germany), as an index for graft tissue damage.
Tissue lipid peroxidation: At the end of reperfusion, tissue samples in all groups were snap frozen in liquid nitrogen and preserved below -80°C. Tissue malondialdehyde (MDA) was extracted at 4°C in 10 mL/g frozen wet-weight of 0.33 mol/L perchloric acid (HClO42), and then neutralized with 2 mol/L potassium hydroxide (KOH). After removing precipitated KClO4, the MDA concentrations were determined using a fluorometric method, as detailed elsewhere[14].
Oxygen consumption: The oxygen consumption of the small bowel tissue was determined as a parameter for the remaining metabolic activity of the grafts. Perfusate samples at 30 min reperfusion were taken both from the SMA inflow and from the portal venous effluent, and their oxygen contents were measured immediately by a pH-blood gas analyzer (ABL 5, Acid-Base Laboratory, Radiometer, Copenhagen, Denmark). The oxygen uptake was calculated from the difference between the both samples and expressed as μL O2 per minute per gram dry-weight, according to the perfusion flow, tissue mass and the solubility coefficient (24 μL of O2 per milliliter buffer).
Perfusate samples at 15 min of reperfusion were also tested for fragmented DNA release using a Cell Death Detection ELISAPLUS Kit (Roche Molecular Biochemicals, Mannheim, Germany), according to the manufacturer’s instruction.
At the end of CS, additional tissue samples (n = 3 each) were perfused with glutaraldehyde and paraformaldehyde solution (2% each in phosphate-buffered saline) through SMA with a constant pressure of 70 cm H2O, then immersed into the same solution for at least 2 d at 4°C. The samples were cut into 0.5 mm slices, post-fixed with OsO4 (osmic acid fixative) and embedded in Epon 812 (Serva, Heidelberg, Germany). Semithin sections were then stained according to the procedure by Richardson et al[15]. Thin sections were thereafter stained with uranyl acetate and lead citrate, and then examined with a Phillips CM 10 electron microscope (Phillips, Eindhoven, The Netherlands).
All results are expressed as mean ± SE from the 7 independent observations, unless otherwise mentioned. Comparisons among the 4 groups were performed by one-way analysis of variance (ANOVA), followed by Tukey-Kramer’s post hoc test. The differences were considered statistically significant when P-values were less than 0.05.
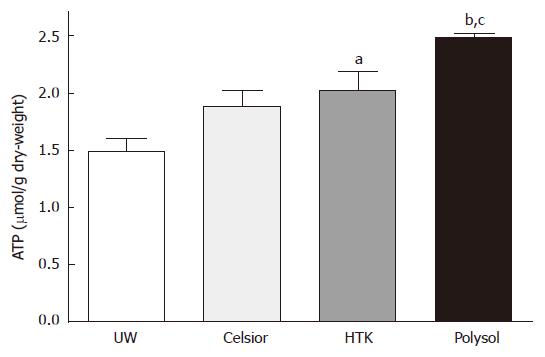
The tissue ATP concentration of the graft after CS and oxygenated reperfusion is regarded as one of the most important parameters reflecting the organ viability. We determined the tissue ATP contents at the end of reperfusion in all groups (Figure 1). The value in Polysol (2.480 ± 0.036 μmol/g dry-weight) were highest, and was significantly higher than UW (1.488 ± 0.119 μmol/g dry-weight, P < 0.001) and in Celsior (1.885 ± 0.143 μmol/g dry-weight, P < 0.05). The value in HTK (2.031 ± 0.156 μmol/g dry-weight) was also significant higher than UW (P < 0.05).
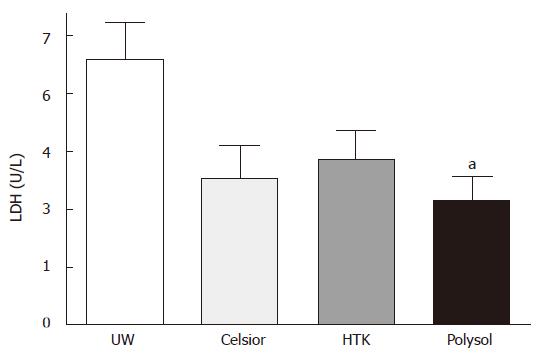
After 18 h CS and 30 min oxygenated reperfusion, LDH release in UW (69 ± 9.4 U/L) was the highest, followed by HTK (43 ± 7.3 U/L) and Celsior (38 ± 8.4 U/L). The release was the lowest in Polysol (32 ± 6.5 U/L) among the 4 groups. Only the value in Polysol reached the statistically significant level versus UW (P < 0.05, Figure 2).
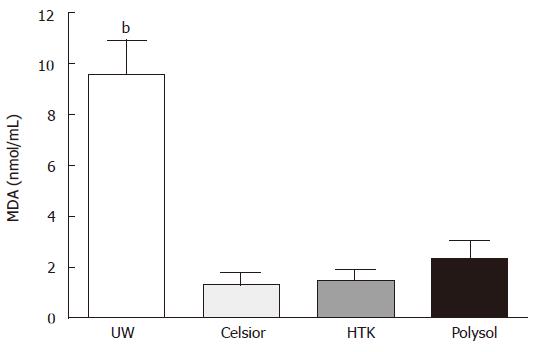
Tissue MDA content in UW was 9.55 ± 1.360 nmol/mL, which was significantly higher than the other 3 groups (P < 0.001 vs the others). The values in Polysol (2.34 ± 0.731 nmol/mL). HTK (1.47 ± 0.452 nmol/mL) and Celsior (1.33 ± 0.460 nmol/mL) were not significantly different (Figure 3).
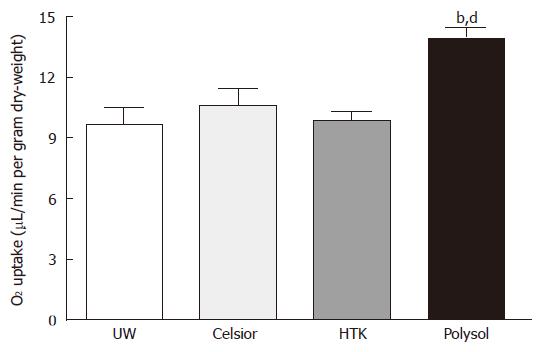
The levels of tissue oxygen uptake were not different among the 3 groups (UW: 9.65 ± 0.83, Celsior: 10.63 ± 0.80, HTK: 9.89 ± 0.46 μL/min per gram dry-weight). The value in Polysol (13.94 ± 0.51 μL/min per gram dry-weight) showed the highest among the 4 groups (Figure 4), reaching the significantly higher level vs Celsior, HTK (P < 0.01) and UW (P < 0.001).
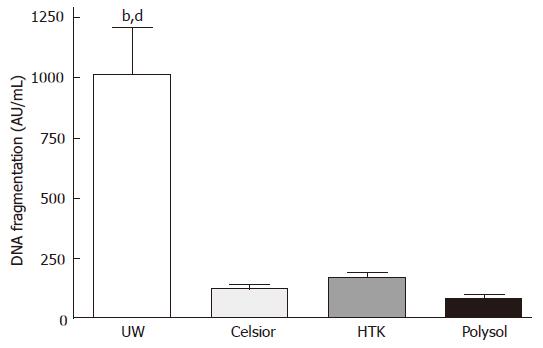
To quantitatively assess the magnitude of apoptosis, we performed a DNA fragmentation ELISA. The highest apoptotic cell death was detected in the UW group (1014 ± 268 AU/mL), which was significantly higher than the other 3 groups (P < 0.01 vs HTK, P < 0.001 vs Celsior and Polysol). The lowest apoptosis was detected in Polysol (81 ± 17 AU/mL), followed by Celsior (123 ± 17 AU/mL) and HTK (172 ± 20 AU/mL). Although statistically not significant to Celsior and HTK, Polysol group showed the lowest values, while 10-fold higher apoptosis was identified in UW (Figure 5).
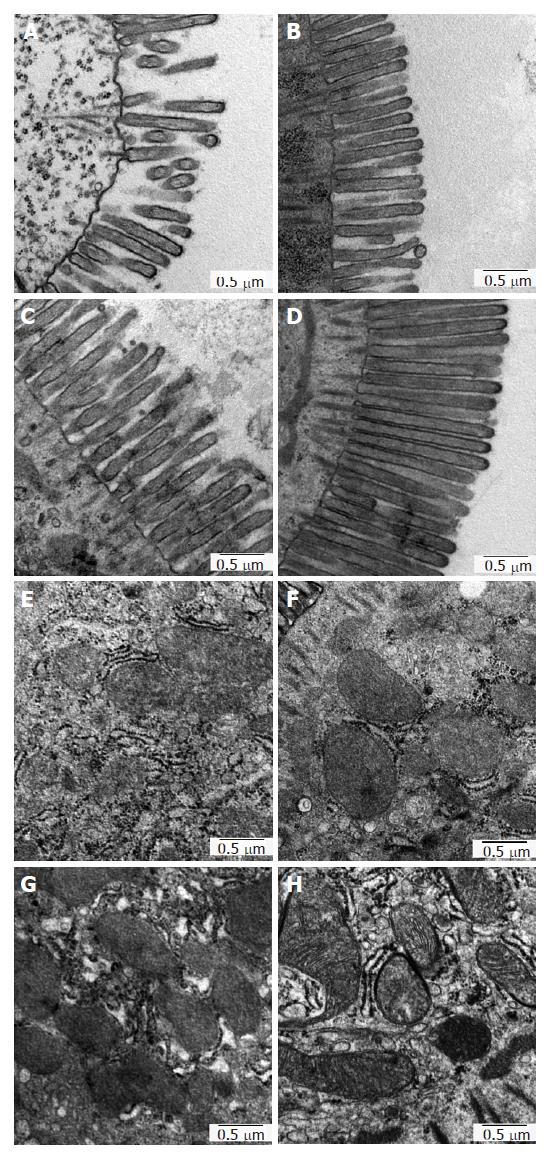
In order to evaluate the graft tissue damage at ultrastructural level, especially focusing on the microvilli formation as well as on the cell organelles in enterocytes, we conducted electron microscopy after 18 h CS, as illustrated in Figure 6.
The microvilli formation in UW was severely damaged, showing sparse and lowered-height of microvilli (Figure 6A). In Celsior, although the microvilli density was relatively better preserved than in UW, the height of microvilli was apparently lowered to the same level in UW (Figure 6B). In HTK, the height of microvilli was better preserved than in UW and in Celsior; however, the breakdown of their apical region was diffusely noted, displaying ragged and jagged formation (Figure 6C). In contrast to the other 3 groups, such deleterious alterations were rarely visible in Polysol, indicating homogeneously preserved microvilli epithelia of the luminal surface (Figure 6D).
With regard to the damage in cell organelles, the mitochondria in UW displayed mild swelling and less electron density of their matrices, in which the cristae were hardly visible (Figure 6E). In Celsior and HTK, such detrimental alterations in mitochondria were also identified, in spite of relatively better-preserved electron density of the ultrastructures (Figure 6F and G). Unlike the other groups, the mitochondria in Polysol kept their oval shape with well-maintained cristae formation (Figure 6H). These findings are thus in good agreement with the results of tissue oxygen consumption and the resulting ATP contents, at the point of mitochondrial damage and function.
Unlike other organs, small bowel transplantation (SBTx) has several characteristic burdens, such as rich lymphoid tissues and large mucosal surface expressing class-2 major histocompatibility antigens. Indeed, the recent achievements in immunosuppressants, including the development of Tacrolimus, had brought a new era also in the management and outcome of clinical SBTx[16]. Thus, uncontrollable immunological rejection, which had long been the main cause of patient death, was superseded by septic complications as the main cause of mortality[1,8]. These events then emphasize another aspect of high hurdles in SBTx: How to preserve the mucosal integrity and the barrier functions during/after organ preservation.
In the present study, we demonstrated that cold storage (CS) of small bowel grafts using Polysol resulted in significantly better quality of graft preservation than the current clinical standard, UW, in all the parameters we tested. Surprisingly, UW exhibited the worst trend of results in most parameters, even compared with other currently available solutions, HTK and Celsior. In particular, morphological integrity of the mucosa, both microvilli formation and cell organelles, such as mitochondria, were significantly better preserved by Polysol, whereas detrimental alterations were apparent in the UW-stored grafts. Although the present study did not elucidate the exact mechanisms of the benefits by Polysol, as well as of the worst results by UW, one possible explanation to this difference is, at least in part, in numerous amino-acid supplementations in Polysol.
Polysol contains up to 21 amino acids. During CS, these amino acids play protective roles in a variety of cellular house keeping processes by catering both for metabolic (energy production) and for synthetic (synthesis of critical molecules) aspects of intestinal metabolism[17,18]. Among them, glutamine, glutamate, and aspartate are crucial for enterocytes as major energy sources[19]. The intestinal glutamine catabolism is a multistep process, resulting in ATP production via the TCA cycle or via the provision of key elements to the nitrogen-carbon backbone of purines. Since the glucose utilization is limited during CS, glutamine supplementation is thought to be effective to maintain cellular ATP level, thus alleviating the tissue damage[20]. Furthermore, because small intestinal mucosa becomes atrophic when the gut is deprived of glutamine, its supplementation surely counteracts the mucosal atrophy during/after cold storage, as manifested by electron microscopy. Glutamic acid also augments energy production in anaerobic conditions[12]. Moreover, glycine is expected to work against oxygen species and stabilize the tertiary protein structure of cell membranes upon reperfusion[21]. Also, glycine can restore cellular ATP levels[22]. As already reported, amino-acid based solutions could provide a better energy storage in the small bowel preservation[23,24].
Another characteristic difference is noticed in the anti-edematous ingredients (Table 1). Different from the others, Polysol contains polyethylene glycol (PEG) that enables both the required oncotic pressure and the relatively low viscosity (1.8 cP). PEG stabilizes lipid membranes and contributes to less membrane permeability[25,26], thus preventing osmotic cell swelling and vascular endothelial damage[27]. Moreover, PEG also act as a OFR scavenger, markedly reducing lipid peroxidation upon preservation/reperfusion process[27,28]. In UW, the colloid used is hydroxyethyl starch (HES), which (5% in UW) causes over 3-fold higher viscosity (5.7 cP), thereby triggering vasoconstriction and endothelial damage, in cooperation with the high potassium concentration[29,30]. Moreover, HES also causes hyperaggregation of erythrocytes, which may result in incomplete, heterogeneous blood-washout of the graft tissue. On the other hand, HTK and Celsior are crystalloid solutions with lower viscosity and lower potassium concentration (Table 1). It was already proven that HTK and Celsior were superior than UW as an initial flushing solution, not only for small bowel but for liver and heart[31,32], mainly due to the lower viscosity of 1.3-1.8 cP. The viscosity of Polysol is also 1.8 cP, and was proven to have the same efficacy to HTK as a flushing solution[33].
Among the parameters we investigated, one of the most remarkable differences is notable in the magnitude of apoptosis. Surprisingly, the apoptosis in the UW-stored grafts was almost 10-fold higher than that in Polysol. Apoptosis affects the permeability of the intestinal mucosa, impairs its barrier function after transplantation, thus leading to BT and endotoxin absorption. As previously demonstrated, apoptosis is a predominant form of cell death in intestinal IRI[34], and is an important predictive factor for primary allograft dysfunction in clinical SBTx[35]. Considering the mechanisms underlying the difference in apoptosis, we focused on the antioxidative properties in each solution. As presented in Figure 4, lipid peroxidation, caused by oxygen free radicals (OFR), in UW group was almost 5-fold higher than the other groups. Polysol has optimized OFR scavenging potential, achieved by supplementation of not only glutathione and allopurinol but also ascorbic acid (vitamin C), selenium, glycine and alpha-tocopherol (vitamin E), all of which were proven to be effective as antioxidants upon organ preservation[36-38]. Celsior and HTK, include relatively high dose of histidine (Table 1), which neutralizes reactive oxygen species and preserves high-energy phosphates[39]. Thus the enhanced antioxidative property of these 3 solutions attenuated OFR-mediated cellular damage upon reperfusion, thereby reducing apoptotic cell death.
Although further investigations in a transplant model are of course required to confirm the findings in this study, this ex vivo experimental setting has several advantages to assess the quality of organ preservation. First, in contrast to a real transplant model, the results from this isolated setting are not influenced from immunological alloreactions, facilitating the precise evaluation for the organ preservation. Taken into account that intestinal mucosa is the main target from the host immune system, because of its high expression of class-2 major histocompatibility antigens, this isolated setting is thought to be feasible to assess the preservation quality itself, without any interference from immunological reactions. Simpler procedures (just organ retrieval, without implantation) and resultant well-reproducible results are also the advantages of this method.
In summary, this is the first report demonstrating a comparison study of the currently available preservation solutions, UW, HTK, Celsior and Polysol, in the efficacy for small bowel preservation. Polysol, a newly developed preservation solution, exhibited the superior quality of small bowel preservation than the current clinical standard, UW after 18 h cold storage. HTK and Celsior also showed better potential in some parameters, however, the both could not reach the over all superiority than UW. Hence in conclusion, Polysol may be a novel suitable alternative for small bowel preservation.
The authors thank Mareike Schulz, Mario Sitzia, Susanne Schulz and Joerg Bedorf for their skillful technical assistance.
S- Editor Zhu LH L- Editor Rippe RA E- Editor Lu W
| 1. | Intestinal Transplant Registry. 2003 International Transplant Registry: 5 year patient survival (data up to 2003). Available from: http//www.intestinaltransplant.org/ITR_Reports/Report_2003/Intestinal Transplant Registry - Final Summary 2003. |
| 2. | Takeyoshi I, Zhang S, Nomoto M, Zhu Y, Kokudo Y, Suzuki T, Hamada N, Nemoto A, Starzl TE, Todo S. Mucosal damage and recovery of the intestine after prolonged preservation and transplantation in dogs. Transplantation. 2001;71:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Biffi R, Privitera G, Andreoni B, Matinato C, Pozzi S, Marzona L, Danza M, Rai P, Tiberio G. Luminal bacterial overgrowth and intestinal translocation in pigs given either cyclosporin A or 15-deoxyspergualin after small bowel transplantation. Eur J Surg. 1995;161:93-96. [PubMed] |
| 4. | Zou Y, Hernandez F, Burgos E, Martinez L, Gonzalez-Reyes S, Fernandez-Dumont V, Lopez G, Romero M, Lopez-Santamaria M, Tovar JA. Bacterial translocation in acute rejection after small bowel transplantation in rats. Pediatr Surg Int. 2005;21:208-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Menger MD. Beyond the epithelial mucosa barrier: monitoring of microvascular perfusion dysfunction in critically endangered intestinal transplants(1,2). J Surg Res. 2006;130:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Kokudo Y, Furuya T, Takeyoshi I, Nakamura K, Zhang S, Murase N, Todo S. Comparison of University of Wisconsin, Euro-Collins, and lactated Ringer's solutions in rat small bowel preservation for orthotopic small bowel transplantation. Transplant Proc. 1994;26:1492-1493. [PubMed] |
| 7. | Leuvenink HG, van Dijk A, Freund RL, Ploeg RJ, van Goor H. Luminal preservation of rat small intestine with University of Wisconsin or Celsior solution. Transplant Proc. 2005;37:445-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Middleton SJ, Jamieson NV. The current status of small bowel transplantation in the UK and internationally. Gut. 2005;54:1650-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Balaz P, Matia I, Jackanin S, Pomfy M, Fronek J, Ryska M. Morphological changes of small bowel graft in Wistar rats after preservation injury. Bratisl Lek Listy. 2004;105:62-64. [PubMed] |
| 10. | Burgmann H, Reckendorfer H, Sperlich M, Spieckermann PG. Comparison of Bretschneider's-HTK and Euro-Collins solution using an in vitro small bowel perfusion model. Transplant Proc. 1996;28:2636. [PubMed] |
| 11. | Hata K, Tolba RH, Wei L, Doorschodt BM, Büttner R, Yamamoto Y, Minor T. Impact of polysol, a newly developed preservation solution, on cold storage of steatotic rat livers. Liver Transpl. 2007;13:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Minor T, Vollmar B, Menger MD, Isselhard W. Cold preservation of the small intestine with the new Celsior-solution. First experimental results. Transpl Int. 1998;11:32-37. [PubMed] |
| 13. | Tolba RH, Akbar S, Müller A, Glatzel U, Minor T. Experimental liver preservation with Celsior: a novel alternative to University of Wisconsin and histidine-tryptophan-alpha-ketoglutarate solutions? Eur Surg Res. 2000;32:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Minor T, Kötting M. Gaseous oxygen for hypothermic preservation of predamaged liver grafts: fuel to cellular homeostasis or radical tissue alteration? Cryobiology. 2000;40:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Richardson KC, Jarett L, Finke EH. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960;35:313-323. [PubMed] |
| 16. | Abu-Elmagd K, Reyes J, Bond G, Mazariegos G, Wu T, Murase N, Sindhi R, Martin D, Colangelo J, Zak M. Clinical intestinal transplantation: a decade of experience at a single center. Ann Surg. 2001;234:404-416; discussion 416-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 266] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | Windmueller HG, Spaeth AE. Respiratory fuels and nitrogen metabolism in vivo in small intestine of fed rats. Quantitative importance of glutamine, glutamate, and aspartate. J Biol Chem. 1980;255:107-112. [PubMed] |
| 19. | Alpers DH. Is glutamine a unique fuel for small intestinal cells? Curr Opin Gastroenterol. 2000;16:155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Olson DW, Fujimoto Y, Madsen KL, Stewart BG, Carle M, Zeng J, Jewell L, Sheasgreen JL, Chong FT, Kneteman NM. Potentiating the benefit of vascular-supplied glutamine during small bowel storage: importance of buffering agent. Transplantation. 2002;73:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M, Bunzendaul H, Bradford B, Lemasters JJ. L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr Metab Care. 2003;6:229-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 270] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 22. | Deters M, Strubelt O, Younes M. Protection by glycine against hypoxia-reoxygenation induced hepatic injury. Res Commun Mol Pathol Pharmacol. 1997;97:199-213. [PubMed] |
| 23. | Salehi P, Zhu JZ, Castillo EG, Avila J, Lakey J, Churchill TA. Preserving the mucosal barrier during small bowel storage. Transplantation. 2003;76:911-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Tsujimura T, Salehi P, Walker J, Avila J, Madsen K, Lakey J, Kuroda Y, Churchill TA. Ameliorating small bowel injury using a cavitary two-layer preservation method with perfluorocarbon and a nutrient-rich solution. Am J Transplant. 2004;4:1421-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Robinson JR. Control of water content of non-metabolizing kidney slices by sodium chloride and polyethylene glycol (PEG 6000). J Physiol. 1971;213:227-234. [PubMed] |
| 26. | Daniel MR, Wakerley CL. Factors influencing the survival of cell monolayers during storage at 4 degrees. Br J Exp Pathol. 1976;57:137-147. [PubMed] |
| 27. | Hauet T, Mothes D, Goujon JM, Carretier M, Eugene M. Protective effect of polyethylene glycol against prolonged cold ischemia and reperfusion injury: study in the isolated perfused rat kidney. J Pharmacol Exp Ther. 2001;297:946-952. [PubMed] |
| 28. | Mack JE, Kerr JA, Vreugdenhil PK, Belzer FO, Southard JH. Effect of polyethylene glycol on lipid peroxidation in cold-stored rat hepatocytes. Cryobiology. 1991;28:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Pearl JM, Laks H, Drinkwater DC, Sorensen TJ, Chang P, Aharon AS, Byrns RE, Ignarro LJ. Loss of endothelium-dependent vasodilatation and nitric oxide release after myocardial protection with University of Wisconsin solution. J Thorac Cardiovasc Surg. 1994;107:257-264. [PubMed] |
| 30. | Mankad P, Slavik Z, Yacoub M. Endothelial dysfunction caused by University of Wisconsin preservation solution in the rat heart. The importance of temperature. J Thorac Cardiovasc Surg. 1992;104:1618-1624. [PubMed] |
| 31. | Müller AR, Nalesnik M, Platz KP, Langrehr JM, Hoffman RA, Schraut WH. Evaluation of preservation conditions and various solutions for small bowel preservation. Transplantation. 1994;57:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Puhl G, Olschewski P, Schöning W, Hunold G, Liesaus HG, Winkler R, Neumann UP, Schubert TE, Schmitz V, Neuhaus P. Low viscosity histidine-tryptophan-ketoglutarate graft flush improves subsequent extended cold storage in University of Wisconsin solution in an extracorporeal rat liver perfusion and rat liver transplantation model. Liver Transpl. 2006;12:1841-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Bessems M, Doorschodt BM, Albers PS, van Vliet AK, van Gulik TM. Wash-out of the non-heart-beating donor liver: a comparison between ringer lactate, HTK, and polysol. Transplant Proc. 2005;37:395-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Ikeda H, Suzuki Y, Suzuki M, Koike M, Tamura J, Tong J, Nomura M, Itoh G. Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium. Gut. 1998;42:530-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 235] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 35. | Oberbauer R, Rohrmoser M, Regele H, Mühlbacher F, Mayer G. Apoptosis of tubular epithelial cells in donor kidney biopsies predicts early renal allograft function. J Am Soc Nephrol. 1999;10:2006-2013. [PubMed] |
| 36. | Albuquerque RG, Sanson AJ, Malangoni MA. Allopurinol protects enterocytes from hypoxia-induced apoptosis in vivo. J Trauma. 2002;53:415-20; discussion 420-1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Yin M, Zhong Z, Connor HD, Bunzendahl H, Finn WF, Rusyn I, Li X, Raleigh JA, Mason RP, Thurman RG. Protective effect of glycine on renal injury induced by ischemia-reperfusion in vivo. Am J Physiol Renal Physiol. 2002;282:F417-F423. [PubMed] |
| 38. | Mutlu-Türkoğlu U, Erbil Y, Oztezcan S, Olgaç V, Toker G, Uysal M. The effect of selenium and/or vitamin E treatments on radiation-induced intestinal injury in rats. Life Sci. 2000;66:1905-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Cai Q, Takemura G, Ashraf M. Antioxidative properties of histidine and its effect on myocardial injury during ischemia/reperfusion in isolated rat heart. J Cardiovasc Pharmacol. 1995;25:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |