Published online Jul 14, 2007. doi: 10.3748/wjg.v13.i26.3598
Revised: November 6, 2006
Accepted: February 14, 2007
Published online: July 14, 2007
AIM: To evaluate the effects of protein deprivation on the myenteric plexus of the esophagus of weanling rats.
METHODS: Pregnant female Wistar rats were divided into 2 groups: nourished (N), receiving normal diet, and undernourished (D), receiving a protein-deprived diet, which continued after birth. At twenty-one days of age, 13 esophagi from each group were submitted to light microscopy and morphometrical analysis employing the NADH diaphorase, NADPH diaphorase and acetylcholinesterase techniques. Three other esophagi from each group were evaluated with transmission electron microscopy (TEM).
RESULTS: In both the NADH- and the NADPH-reactive mounts, the neurons of the N mounts were more intensely stained, while in the D esophagi only the larger neurons were reactive. Many myenteric neurons of N were intensely reactive for AChE activity but only a few neurons of D exhibited these aspects. Ultrastructural analysis revealed that the granular reticulum of N showed large numbers of ribosomes aligned on the outer surface of its regularly arranged membrane while the ribosomes of D were disposed in clusters. The chromatin was more homogeneously scattered inside the neuron nucleus of N as well as the granular component of the nucleolus was evidently more developed in this group. Statistically significant differences between N and D groups were detected in the total estimated number of neurons stained by the NADPH technique.
CONCLUSION: The morphological and quantitative data shows that feeding with protein-deprived diet in 21-d old rats induces a delay in the development of the myenteric neurons of the esophagus.
- Citation: Liberti EA, Fontes RB, Fuggi VM, Maifrino LB, Souza RR. Effects of combined pre- and post-natal protein deprivation on the myenteric plexus of the esophagus of weanling rats: A histochemical, quantitative and ultrastructural study. World J Gastroenterol 2007; 13(26): 3598-3604
- URL: https://www.wjgnet.com/1007-9327/full/v13/i26/3598.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i26.3598
The effect of protein malnutrition has been studied recently in the enteric nervous system (ENS), particularly on the myenteric plexus of the infra-diaphragmatic part of the digestive tract and varying results have emerged. It has been previously determined that in the jejunum of rats it results in a 27% decrease in the number of myenteric neurons[1]. The colon of protein-deprived adult rats also exhibited 13% neuronal loss[2], while in young rats the total number of neurons was not reduced[3]. On the other hand, in the small intestine of protein-deprived young rats the estimated total number of myenteric neurons actually increases[4] and a low-protein diet leads to a greater density of myenteric neurons in duodenum[5] and jejunum of adult rats[6].
The normal myenteric plexus of the esophagus has been the object of studies employing different anatomical techniques in several animals and stages of both embryonic and adult life, including the opossum[7], cat[8,11], mice, rabbits, sheep[9], guinea-pig[9,10], monkey[11], rat[12] and several others including the man[13]. However, morphological information on the effects of malnutrition on the myenteric plexus of this organ is still scarce. Therefore, the objective of this paper was to evaluate the effects of a severe, combined pre- and postnatal protein deprivation on the myenteric neurons in the esophagus of young rats, employing different anatomical techniques.
The study was conducted according to the current legislation on animal experiments of the Biomedical Science Institute of the University of Sao Paulo and approved by its Ethics Committee. Young male and female Wistar rats (200-240 g body weight) were mated during a period from seven to ten days. After conception, the females were placed in individual cages and maintained under standard conditions at 21°C with a 12-h light/dark cycle. During pregnancy, female rats were randomly selected into nourished (N) and undernourished (D) groups. The N rats received standard AIN-93G[14] normal protein diet and the undernourished (protein-deprived) mothers (D) received the modified AIN-93G low-protein diet (5% casein) (Rhoster, São Paulo, Brazil). Both groups were supplied with water ad libitum.
Following birth, animals from both groups (N and D) received their respective diets. At the end of the weanling period (21 d) male animals were selected for examination. After weighing, they were killed with 3% sodium pentobarbital (Fontoveter, São Paulo, Brazil) and had the esophagus entirely removed and processed using the following techniques.
Five animals from each group were selected for this technique. Esophagi were initially washed in Krebs solution, ligated with cotton threads at its extremities and filled through a syringe needle in its lumen until slightly distended. After incubation in Krebs solution at room temperature for 15-30 min, the specimens were transferred to a permeabilizing agent (0.3% Triton-X in Krebs solution) for 60 s and then submitted to three 10-min washings in Krebs solution, as described earlier[15].
Esophagi were then incubated for 60 min at 20°C in 20 mL of a medium containing 0.5 mg/mL nitro blue tetrazolium (Sigma-Aldrich) in distilled water (5 mL), 0.1 mol/L sodium phosphate buffer (5 mL, pH 7.3), distilled water (10 mL) and 0.5 mg/mL ®-nicotinamide adenine dinucleotide (reduced form). The reaction was stopped by immersion in 10% buffered formalin solution in which the viscera were fixed (24 h minimum). The esophagi were next longitudinally opened and, using a puncture device, three circular fragments of 20 mm2 each were obtained from the oral, medium and aboral regions of each specimen. The mucosa and submucosa layers of these fragments were removed and the specimens were thoroughly washed in distilled water. Finally, whole-mount preparations were laid in glycerol on a microscope slide and sealed with Entellan (Merck KGaA, Darmstadt, Germany).
Five esophagi from the N and D groups each were selected for this study. After initial washing with Krebs solution, specimens were ligated at their extremities and filled with 4% paraformaldehyde in phosphate buffer (pH 7.4) and immersed in this same substance for 2 h at 4 degrees Celsius as previously described[16,17]. They were next washed in phosphate buffer for 2.5 h and incubated in a medium containing 1 mg/mLβ-NADPH (reduced form, Sigma) in phosphate buffer for 35 min. Thereafter the three circular fragments (oral, medium and aboral) were obtained from each esophagus and whole-mounts prepared as above-mentioned.
In three esophagi from each group (N and D) AChE activity was demonstrated with its direct coloring method as described elsewhere[18,19]. The specimens were filled with 4% paraformaldehyde in phosphate buffer (pH 7.4), ligated at their oral and aboral limits and immersed in the same fixative solution for 2 h at 4°C. After this period the esophagi were opened and their oral, medium and aboral fragments (obtained as above-mentioned) were maintained overnight at 4°C in a solution with hyaluronidase (Hialozime®; Apsen), Krebs solution and tetraisopropylpyrophosphramide (iso-OMPA; Sigma). The fragments were then removed, washed in Krebs solution and once again incubated overnight at 4°C in a second solution containing 50% of solution A plus 0.17 mol/L acetylthiocholine iodide, 0.1 mol/L phosphate buffer (pH 7.1), 100 mmol/L sodium citrate, 30 mmol/L cupric sulfate, 5 mmol/L kalium ferricyanatum and 0.3% Triton-X100. The mucosal and submucosal layers were removed and the fragments dehydrated in an increasing alcohol series (70-100 degrees), immersed in benzene for 20 min and mounted on microscope slides with Entellan (Merck KGaA, Darmstadt, Germany).
Ultrastructural features of the myenteric neurons were observed in fragments from three other N and D esophagi which were fixed for 6 h in 3% glutaraldehyde in Millonig's buffer, pH 7.2-7.4, containing 0.25% tannic acid, as previously described[20]. The specimens were post-fixed in a 2% osmium tetroxide solution and embedded in adhesion medium. Ultra-thin sections were stained with uranyl acetate-lead citrate[21] and examined in a JEOL JEM-1010 (Jeol, Tokyo, Japan) electron transmission microscope.
The neuronal density and the profile areas of the nerve cell bodies were measured by examining the whole-mount preparations stained with the NADH and NADPH reactions under a binocular microscope at magnification of × 400. For each esophagus all neurons present in the oral, medium and aboral fragments were counted. The profiles of 50 random nerve cell perikarya from each specimen were obtained on a semiautomatic device for morphometry analysis. The data were expressed as means ± SE and compared by analysis of variance (ANOVA), Student's t-test and Duncan's test for multiple comparisons, as appropriate. The level of significance was set at P < 0.05[22].
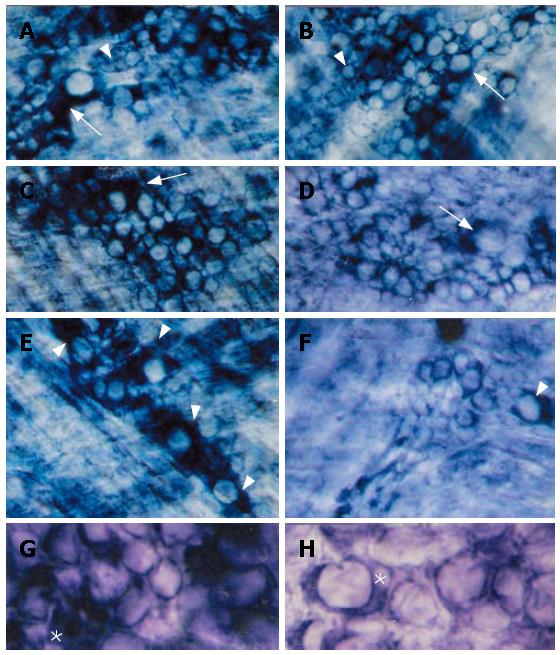
For both N and D groups, no significant differences were observed among the oral, medium and aboral fragments in all histochemical reactions here performed. In relation to the NADH-reactive neurons, a predominantly intense and homogeneous staining pattern was observed in large, medium and most of the small myenteric neurons of normally-fed feed animals (N) (Figure 1A, C, E and G). In the protein-deprived animals (D), large neurons were generally more weakly-stained than those of the N group (Figure 1B, D, F and H).
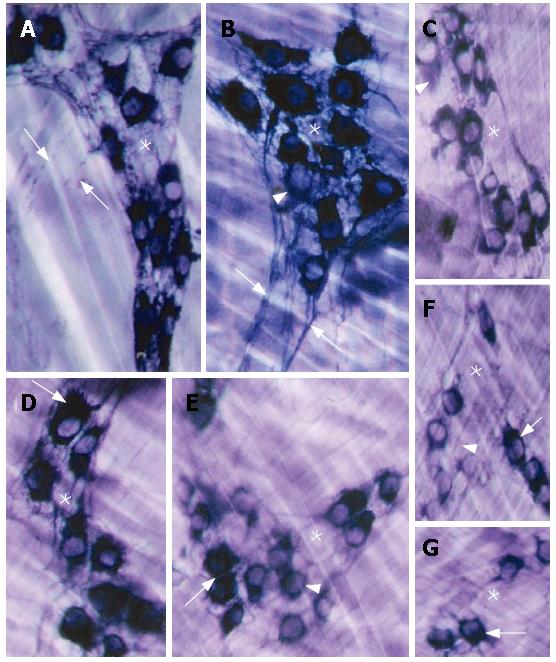
The NADPH reaction strongly stained both myenteric neurons and the ganglion network of the esophagi of the N animals. Only a few neurons were weakly stained in this group, as shown in Figure 2A, B and D. In contrast, although some NADPH-reactive neurons were also detected in the D group, they were generally less reactive (Figure 2C, F and G). Furthermore, a thin network of neuronal processes was well evidenced inside the ganglia of N esophagi, it contours reactive neurons and can be seen in the space between NADPH reactive and non-reactive neurons (Figure 2A, B and D). In the ganglia of D, however, the thin network of neuronal processes was not clearly defined, especially in the space between neurons, suggesting in some cases a reduction in their number (Figure 2C and G). The axonal processes were evidenced in both N and D groups but much defined in ganglia of N (Figure 2A and B).
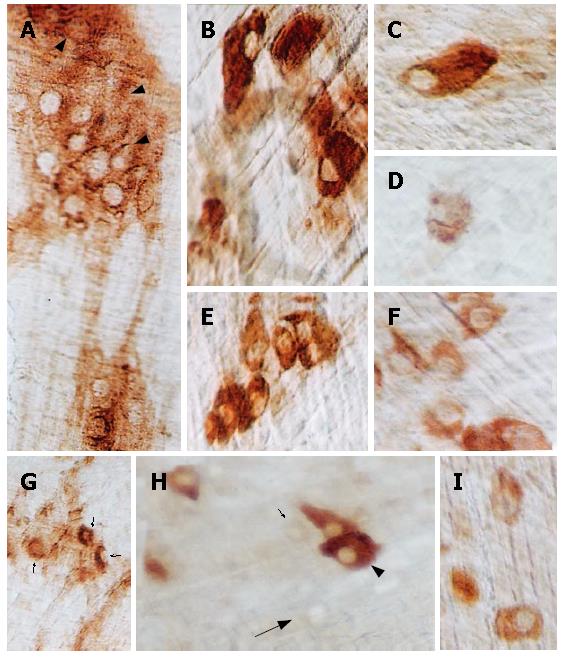
Many myenteric neurons in N ganglia were intensely reactive for AChE activity, with a homogenous cytoplasmic staining pattern (Figure 3A-C, E). Neurons exhibiting this staining pattern, on the other hand, were extremely rare in D ganglia (Figure 3G and H). In this second group, the characteristic staining pattern was the presence of poorly- to moderately-reactive neurons and the shadows of these cells in the wide, blank spaces inside the ganglia (Figure 3D, F-I).
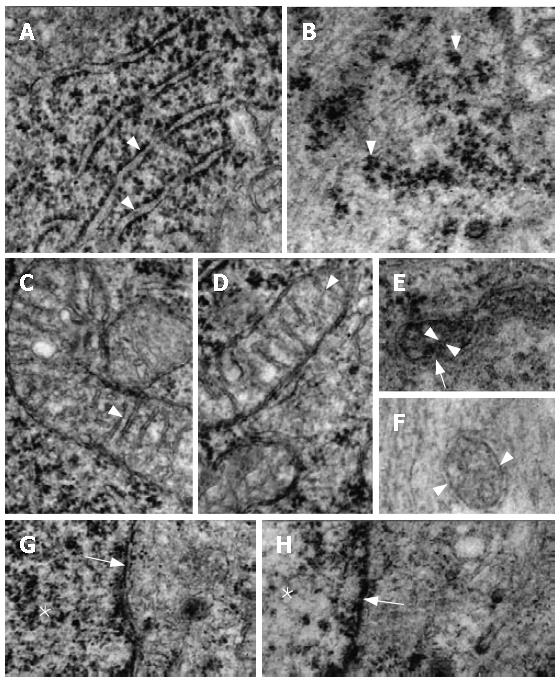
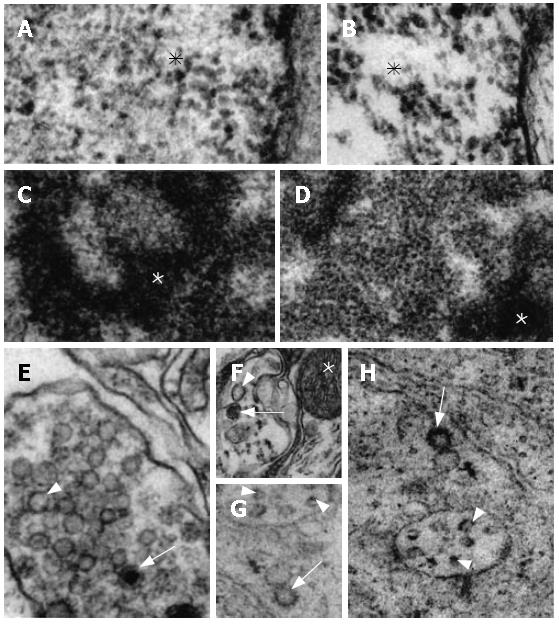
The ultrastructural characteristics of the N myenteric neurons were clearly distinct from those of the D group. Thus, the granular reticulum of N neurons exhibited large numbers of ribosomes aligned on the outer surface of its regularly-arranged membrane (Figure 4A). In the D neurons its ribosomes were disposed in clusters and the membrane of the reticulum was only poorly evidenced (Figure 4B). The mitochondria of N was composed of its characteristic double membrane, with the infoldings of its inner layer arranged in the usual transversal cristae (Figure 4C and D). In general, these organelles in the D neurons were more electron-dense but their double membrane was preserved. However, most of their cristae were not evidenced and several of them had an oblique orientation (Figure 4E and F). The nuclear membrane had a normal aspect in both N and D groups (Figure 4G and H). The chromatin was more homogeneously scattered inside the neuronal nucleus of the N group (Figures 4G and 5A) while in the neurons of the D group its distribution was irregular, with clear spaces between the accumulated electron-dense material (Figures 4H and 5B). The ultrastructural aspect of the nucleolus was remarkably different between the N and D groups, especially concerning the dense part of the granular component, which was evidently more developed in N than in D (Figure 5C and D). Granular and agranular vesicles, as well as large and small ones, were observed in both N and D groups but they were more defined and electron-dense in the animals of the N group (Figure 5E-H).
The data (mean ± SD) relative to the area of the esophagus, neuronal density, total number of neurons and neuronal profiles for the N and D groups concerning both NADH and NADPH reactions are expressed in Table 1. Statistically significant differences between the N and D groups were detected only in the total esophageal in both NADH and NADPH reactions and in the total estimated number of NADPH-reactive neurons.
| NADH | NADPH | |||
| N21 | D21 | N21 | D21 | |
| Esophageal area (mm2) | 191.6 ± 4.0a | 145.4 ± 5.8 | 198.2 ± 9.3a | 150.2 ± 9.9 |
| Neuronal density (/mm2) | 2.7 ± 0.7 | 2.5 ± 1.1 | 2.5 ± 0.3 | 1.7 ± 0.8 |
| Total number of neurons | 521 ± 143 | 372 ± 168 | 489 ± 60a | 257 ± 114 |
| Neuronal profile (μm2) | 444.1 ± 86.3 | 391.1 ± 50.1 | 475.7 ± 82.3 | 399.0 ± 49.0 |
Neuronal area profiles did not differ significantly between the two groups. Approximately 50% of N NADH-stained neurons had their perikarya ranging from 200 to 400μm2 and another 35% ranging from 400 to 700μm2. Likewise, in the D group 62% of NADH-positive neurons ranged from 200 to 400μm2 and 30% from 400 to 700μm2. Accordingly, most of the perikarya of NADPH-positive neurons in N and D groups ranged from 300-600μm2 (respectively 75% and 71%).
Although morphological changes in the offspring of different animal species determined by malnutrition have been detected in organs such as the kidney[23], skin[24] and striated muscle[25], it has been reported that, in spite of a reduction in organ weight and size, the gross structure of the lungs, heart and liver was not remarkably altered[26-28]. Macroscopically, there is an indication that in the central nervous system (CNS) that cortical thickness itself is more affected by malnutrition than the cerebrum as a whole[29]. Nevertheless, if the severe malnutrition causes nerve cell loss in some regions of the CNS (e.g., dentate gyrus)[30], the cerebral cortex seems protected from neuronal loss[31]. Previous data concerning malnutrition on the ENS of rats is also conflicting: there is evidence showing that it determines a decrease in the number of myenteric neurons in the jejunum[1], a increase of these cells in the small intestine as a whole[4] or yet does not alter the number of myenteric neurons in the colon of protein-deprived young animals[3].
In spite of the N animals exhibiting a larger total esophageal area, nerve cells were relatively sparse in the myenteric plexus of both N and D groups, such as had already been demonstrated in other animal species[32]. On the NADH specimens, myenteric ganglia of the D samples retained their usual structural arrangement; structural alterations and open spaces suggesting neuronal loss, as described in the colon of aged and Trypanossoma cruzi-infected animals, was not observed[33,34], nor was the abnormal distribution of ganglia as reported in the atretic esophagus of the rat fetus[35]. Although in this group (D) the cytoplasm of many neurons had diffuse or less intense staining, none of the characteristic nuclear changes of dying cells were present. In fact, this may reflect the varying maturity of the myenteric neurons, as observed in the myenteric plexus of rats during early postnatal development where different intensities of cuprolinic blue staining was detected by other researchers[12]. This aspect is reinforced by the relatively normal ultrastructural features of mitochondria in neurons of the D group as compared to the peculiar characteristics of these organelles in apoptotic cells, such as circular cristae and in- or evaginations of the mitochondrial outer membrane were not detected[36]. On the contrary, some of the ultrastructutural aspects of D neurons, including the disarranged granular reticulum and the smaller density of the granular part of the nucleolus, clearly suggest a delay in the development of these cells determined by the protein deprivation. The quantitative data of NADH-reactive neurons resembles the findings on the colon of protein-deprived young animals[3], in that the total estimated number of neurons in the esophagus was not statistically different between N and D groups. This is also in line with the data for the proximal portion of the colon, where another group could not demonstrate a difference in neuronal number between undernourished and nourished rats[37].
As for the NADPH-positive neurons, a reduction in their number was verified in the esophagus of D animals. This condition is similar to that described in the esophagus of aging rats where they were also reduced[11]. However, the high proportion of NADPH-positive neurons among total neurons in N group (approximately 90%) is maintained in D group (approximately 70%) corroborating the results observed in the myenteric plexus of the esophagus of young and aging rats (64%-89%)[38].
It has been postulated that the enteric neuronal control of the gastrintestinal motility is mediated by excitatory (mainly colinergic) and inhibitory (mainly adrenergic) neurotransmitters. Some of the previously called "non-colinergic and non-adrenergic" neurotransmitters such as nitric oxide (NO) are also involved. A simplified model is based on acetylcholine (AChE) initiating muscular contraction while NO acts in the muscular relaxation phase[39,40]. Under normal conditions there is a harmonious interplay between excitatory and inhibitory neurotransmitters on gastrintestinal motility. The predominance of poorly to moderately AChE-reactive myenteric neurons in the D group associated with the decrease in the number of NADPH-positive neurons are suggestive of a developmental delay of these myenteric neurons; one may assume that this condition determines a transitory neurotransmitter imbalance, thus altering the motility of the esophagus. Electrophysiological studies may contribute to our understanding of whether the myenteric neurons in the esophagus of young rats are similar to that of the the ileum, duodenum or proximal colon of adult animals[41] or if the electrophysiological characteristics of these cells in D are different from that of N animals. The smaller electron-density of both large and small granular vesicles points out to a substantially important difference.
In summary, the structural and functional effects of malnutrition remain controversial, further because they are dependent on other factors as intensity and duration, which were not addressed in this study, but it appears to bear a direct relation to which life stage it takers place in. Continuing research on its effects on the myenteric neurons and particularly on malnourished animals after re-nutrition may elucidate if the ENS is able to recover from this insult later in life through neuronal plasticity phenomena.
To the Brazilian National Research Council (CNPq) for supporting Libert EA as career investigator.
Malnutrition induces several changes on the digestive tract of both animals and humans but its effects on myenteric plexuses have been poorly described, particularly in the esophagus.
Neuronal loss or atrophy may lead to motility, secretive and absorptive deficiencies with possible clinical implications.
Existing literature shows that the myenteric plexus undergoes adaptive changes in a variety of situations. Underuse, aging and malnutrition, among other stimuli, may induce neuronal atrophy or loss and morphological alterations that are not always reversible. These may correspond to the morphological substrate of physiological changes that ultimately impair the normal functioning of the GI tract.
Our article convincingly shows that pre- and post-natal protein deprivation induces profound morphological changes on the myenteric plexus of the esophagi of weanling rats indicative of severe developmental delay. These alterations may explain motility deficiencies that malnourished human neonates often develop and thus provide a morphological basis to better understand and treat the effects of protein deprivation on the GI tract of humans as well.
The manuscript reports the results from an investigation of the characterizations of myenteric neurons in protein deprivated rats. The authors conclude that the morphological and quantitative data shows that feeding with protein-deprivated diet induces a delay in the development of the myenteric neurons of the esophagus. These results are of interest and supported by the data. The results are well-presented too.
S- Editor Liu Y L- Editor Di Mari JF E- Editor Wang HF
| 1. | Santer RM, Conboy VB. Prenatal undernutrition permanently decreases enteric neuron number and sympathetic innervation of Auerbach's plexus in the rat. J Anat. 1990;168:57-62. [PubMed] |
| 2. | Sant'ana Dde M, Miranda Neto MH, de Souza RR, Molinari SL. Morphological and quantitative study of the myenteric plexus of the ascending colon of rats subjected to proteic desnutrition. Arq Neuropsiquiatr. 1997;55:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Castelucci P, de Souza RR, de Angelis RC, Furness JB, Liberti EA. Effects of pre- and postnatal protein deprivation and postnatal refeeding on myenteric neurons of the rat large intestine: a quantitative morphological study. Cell Tissue Res. 2002;310:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Brandão MCS, De Angelis RC, De Souza RR, Froes LB Liberti EA. Effects of pre- and postnatal protein energy deprivation on the myenteric plexus of the small intestine: a morphometric study in weanling rats. Nut Res. 2003;23:215-223. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Natali MMR, Miranda-Neto MH, Orsi MA. Morphometry and quantification of the myenteric neurons of the duodenum of adult rats fed with hypoproteic chow. Int J Morphol. 2003;21:273-277. |
| 6. | Zanin ST, Molinari SL, Sant'Ana Dde M, de Miranda Neto MH. NADH-diaphorase positive neurons of the jejunum of disnurtured adult rats (Rattus norvegicus): quantitative aspects. Arq Neuropsiquiatr. 2003;61:650-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Christensen J, Robison BA. Anatomy of the myenteric plexus of the opossum esophagus. Gastroenterology. 1982;83:1033-1042. [PubMed] |
| 8. | Lolova I, Itsev D. Prenatal development of the myenteric plexus in cat stomach. Acta Physiol Pharmacol Bulg. 1983;9:63-73. [PubMed] |
| 9. | Gabella G, Trigg P. Size of neurons and glial cells in the enteric ganglia of mice, guinea-pigs, rabbits and sheep. J Neurocytol. 1984;13:49-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Morikawa S, Komuro T. Distribution of myenteric NO neurons along the guinea-pig esophagus. J Auton Nerv Syst. 1998;74:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Rodrigo J, Uttenthal LO, Peinado MA, Esteban FJ, Fernández AP, Serrano J, Martínez de Velasco J, Santacana M, Bentura ML, Martínez-Murillo R. Distribution of nitric oxide synthase in the esophagus of the cat and monkey. J Auton Nerv Syst. 1998;70:164-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Schäfer KH, Hänsgen A, Mestres P. Morphological changes of the myenteric plexus during early postnatal development of the rat. Anat Rec. 1999;256:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | de Souza RR, de Carvalho CA, Liberti EA, Fujimura I. A quantitative study on the myenteric plexus of the distal end of the human esophagus. Gegenbaurs Morphol Jahrb. 1988;134:565-574. [PubMed] |
| 14. | Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939-1951. [PubMed] |
| 15. | Gabella G. Detection of nerve cells by a histochemical technic. Experientia. 1969;25:218-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Christensen J, Fang S. Colocalization of NADPH-diaphorase activity and certain neuropeptides in the esophagus of opossum (Didelphis virginiana). Cell Tissue Res. 1994;278:557-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Santer RM. Survival of the population of NADPH-diaphorase stained myenteric neurons in the small intestine of aged rats. J Auton Nerv Syst. 1994;49:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Karnowsky MJ, Roots LA. A "direct-coloring" thiocholine method for cholinesterases. J Histochem Cytochem. 1964;12:219-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2604] [Cited by in RCA: 2604] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 19. | Baker DG, McDonald DM, Basbaum CB, Mitchell RA. The architecture of nerves and ganglia of the ferret trachea as revealed by acetylcholinesterase histochemistry. J Comp Neurol. 1986;246:513-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Cotta-Pereira G, Rodrigo FG, David-Ferreira JF. The use of tannic acid-glutaraldehyde in the study of elastic and elastic-related fibers. Stain Technol. 1976;51:7-11. [PubMed] |
| 21. | Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17689] [Cited by in RCA: 17252] [Article Influence: 639.0] [Reference Citation Analysis (0)] |
| 22. | Zar JH. Biostatistical analysis. 2nd ed. New Jersey: Prentice-Hall 1984; 2801. |
| 23. | Zeman FJ. Effect of protein deficiency during gestation on postnatal cellular development in the young rat. J Nutr. 1970;100:530-538. [PubMed] |
| 24. | Lansdown AB. Epidermal differentiation in normal and growth-retarded infants: studies in two animal models and in human babies. Br J Dermatol. 1978;99:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | el Haj AJ, Lewis SE, Goldspink DF, Merry BJ, Holehan AM. The effect of chronic and acute dietary restriction on the growth and protein turnover of fast and slow types of rat skeletal muscle. Comp Biochem Physiol A Comp Physiol. 1986;85:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Wigglesworth J. Experimental growth retardation in the foetal rat. J Pathol Bacteriol. 1964;88:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 222] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 27. | Naeye RL. Cardiovascular abnormalities in infants malnourished before birth. Biol Neonat. 1965;8:104-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Shrader RE, Zeman FJ. Skeletal development in rats as affected by maternal protein deprivation and postnatal food supply. J Nutr. 1973;103:792-801. [PubMed] |
| 29. | Clark GM, Zamenhof S, Van Mathens E, Grauel L, Kruger L. The effect of prenatal malnutrition on dimensions of cerebral cortex. Brain Res. 1973;54:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Bedi KS. Effects of undernutrition during early life on granule cell numbers in the rat dentate gyrus. J Comp Neurol. 1991;311:425-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Tolley LK, Bedi KS. Undernutrition during early life does not affect the number of granule cells in the rat olfactory bulb. J Comp Neurol. 1994;348:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Christensen J, Rick GA, Robison BA, Stiles MJ, Wix MA. Arrangement of the myenteric plexus throughout the gastrointestinal tract of the opossum. Gastroenterology. 1983;85:890-899. [PubMed] |
| 33. | Maifrino LB, Liberti EA, Watanabe I, De Souza RR. Morphometry and acetylcholinesterase activity of the myenteric neurons of the mouse colon in the chronic phase of experimental Trypanosoma cruzi infection. Am J Trop Med Hyg. 1999;60:721-725. [PubMed] |
| 34. | Gomes OA, de Souza RR, Liberti EA. A preliminary investigation of the effects of aging on the nerve cell number in the myenteric ganglia of the human colon. Gerontology. 1997;43:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 130] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Cheng W, Bishop AE, Spitz L, Polak JM. Abnormal enteric nerve morphology in atretic esophagus of fetal rats with adriamycin-induced esophageal atresia. Pediatr Surg Int. 1999;15:8-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Baumgart E, Vanhorebeek I, Grabenbauer M, Borgers M, Declercq PE, Fahimi HD, Baes M. Mitochondrial alterations caused by defective peroxisomal biogenesis in a mouse model for Zellweger syndrome (PEX5 knockout mouse). Am J Pathol. 2001;159:1477-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 172] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 37. | Leite-Mello EV, Stabille SR, Miranda Neto MH. Effect of maternal protein deprivation on morphological and quantitative aspects of the myenteric plexus neurons of proximal colon in rats. Arq Neuropsiquiatr. 1997;55:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Hirsch DP, Holloway RH, Tytgat GN, Boeckxstaens GE. Involvement of nitric oxide in human transient lower esophageal sphincter relaxations and esophageal primary peristalsis. Gastroenterology. 1998;115:1374-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 117] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | McKirdy HC, McKirdy ML, Lewis MJ, Marshall RW. Evidence for involvement of nitric oxide in the non-adrenergic non-cholinergic (NANC) relaxation of human lower oesophageal sphincter muscle strips. Exp Physiol. 1992;77:509-511. [PubMed] |
| 40. | Furness JB, Li ZS, Young HM, Förstermann U. Nitric oxide synthase in the enteric nervous system of the guinea-pig: a quantitative description. Cell Tissue Res. 1994;277:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Lomax AE, Sharkey KA, Bertrand PP, Low AM, Bornstein JC, Furness JB. Correlation of morphology, electrophysiology and chemistry of neurons in the myenteric plexus of the guinea-pig distal colon. J Auton Nerv Syst. 1999;76:45-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |