Published online Jun 21, 2007. doi: 10.3748/wjg.v13.i23.3253
Revised: April 2, 2007
Accepted: April 11, 2007
Published online: June 21, 2007
This case report describes a rare complication of a left ventricular assist device (LVAD). A patient with ischemic cardiomyopathy had an LVAD placed due to intractable congestive heart failure following a large anterior myocardial infarction. The patient developed chronic bacteremia and multiple septic episodes. A gastric endoscopy revealed perforation of the anterior wall of the stomach by the LVAD. Gastric acid related erosions were present on the metallic surface suggesting prolonged exposure. This is the second case report of this rare complication and the first case report of a subacute course.
- Citation: Yannopoulos D. Subacute gastric perforation caused by a left ventricular assist device. World J Gastroenterol 2007; 13(23): 3253-3254
- URL: https://www.wjgnet.com/1007-9327/full/v13/i23/3253.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i23.3253
Left ventricular assist devices (LVAD) are used frequently for patients with congestive heart failure that fail maximum therapy. They are used either as a bridge to cardiac transplantation or as a destination therapy. The body of the main pump is usually placed in the abdominal cavity. This case report describes a rare complication of subacute gastric perforation caused by a left ventricular assist device.
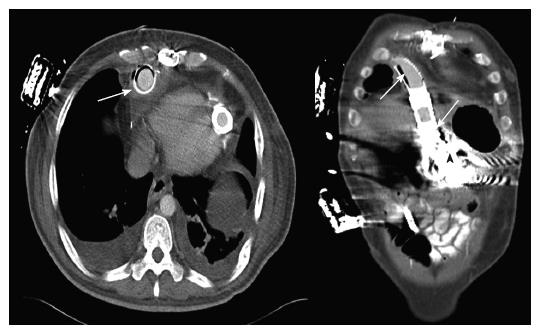
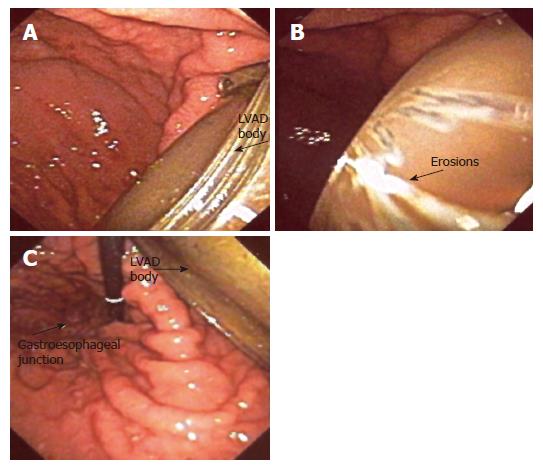
A 51 years old, diabetic patient was transferred to the University of Minnesota Medical Center where a left ventricular assist device (Heart Mate I) was implanted intra abdominally due to persistent cardiogenic shock after an anterior ST elevation myocardial infarction. The patient was also treated with a coronary artery bypass graft with a saphenous vein graft to the posterior descending coronary artery. His peak troponin I was measured at 137 mcg/L. His immediate post operative course was complicated by compartment syndrome of the left lower extremity requiring fasciotomy. This was thought to be secondary to an occluded left femoral-popliteal graft, which had been placed in 1998. Shortly thereafter the patient became septic with gram positive bacteremia. Over the next 2 mo, his health declined and he had multiple septic episodes. They were all treated with intravenous antibiotics. The lower extremity wound was thought to be the cause of his bacteremic events and a left below the knee amputation was performed. The febrile episodes persisted and multiple blood cultures revealed Candida glabrata and Group D Enterococcus vancomycin resistance. The same organisms were isolated from his LVAD drive line insertion site. Despite treatment with antibiotics, blood cultures remained positive. A right side empyema developed and subsequently required thoracostomy. The surgery led to multiple recurrences of hemothoraces, and mechanical ventilation. During his prolonged hospital course efforts to improve nutrition by oral and nasogastric tube feeding resulted in high residual gastric volumes. That was thought to be due to diabetic gastroparesis. A decision was made to improve his nutritional status in preparation for possible surgical explantation of his LVAD which was thought to be contributing to his chronic bacteremias. A CT of the abdomen and chest was performed. There was evidence of air within the outflow cannula of the LVAD device. The air was intraluminal as well as extraluminal. The air was located just above the metal to dacron conversion within the anterior right chest. A follow-up study obtained with technetium-labeled white blood cells indicated that this was an area that had significant white blood cell accumulation consistent with infection (Figure 1). The patient had no abdominal complaints and his abdominal exam was only significant for mild tenderness. Video assisted gastric endoscopy was performed for placement of a duodenal feeding tube and revealed direct exposure of the LVAD in the anterior superior part of the stomach with no communication with the peritoneal cavity. There were gastric acid erosions on the LVAD metallic surfaces suggesting prolonged exposure (Figure 2).
Due to the patient’s ongoing sepsis with deteriorating hemodynamic status requiring vasopressors a family conference was organized and withdrawal of care was decided.
This case report represents the second case of LVAD gastric perforation[1]. Although the first case described was an acute event resulting in immediate death, in our case it is difficult to assess the timing of events. At the time of abdominal CT scan, where air was noticed in the LVAD, air was also noted around the body of the LVAD. Unfortunately, the artifact made it impossible to determine if the device was in the stomach. Gastric enlargement and gastroparesis due to diabetes must have played a role in this event. The patient’s size was within the accepted range for the intra-abdominal LVAD placement (182 cm and 87 kg). These two reported cases show that gastric perforation is a rare, but real complication of LVAD therapy and it should be included in the differential diagnosis of sepsis in this patient population with or without localizing symptoms.
S- Editor Liu Y L- Editor Alpini GD E- Editor Ma WH
| 1. | Hou JK, Hampel H, Lukens FJ. Gastric ulceration and perforation as a complication of a left ventricular assist device. Gastrointest Endosc. 2005;61:629-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |