Published online Jun 7, 2007. doi: 10.3748/wjg.v13.i21.3009
Revised: April 15, 2007
Accepted: April 26, 2007
Published online: June 7, 2007
AIM: To explore the prognostic variables in rectal cancer patients undergoing curative total mesorectal excision and the effect of postoperative chemotherapy in advanced rectal cancer.
METHODS: A total of 259 consecutive rectal cancer patients treated with curative total mesorectal excision between 1999 and 2004 were collected. p53, p21, PCNA, and CD44v6 were examined using immunohistochemistry (IHC). The correlation between clinicopathological or molecular variables and clinical outcomes, including local recurrence, metastasis, disease-free survival and overall survival, was analyzed.
RESULTS: The median follow-up was 44 mo. Five-year survival rates and 5-year disease free survival rates were 75.43% and 70.32%, respectively. Multi-analysis revealed TNM staging, preoperative CEA, and CD44v6 level were independent risk factors predicting overall survival or disease free survival. The hazard ratio of peroperative CEA was 2.65 (95% CI 1.4-5) and 3.10 (95% CI 1.37-6.54) for disease free survival and overall survival, respectively. The hazard ratio of CD44v6 was 1.93 (95% CI 1.04-3.61) and 2.21 (95% CI 1.01-4.88) for disease free survival and overall survival, respectively. TNM staging was the only risk factor predicting local recurrence. Postoperative chemotherapy without radiotherapy did not improve patients’ outcome.
CONCLUSION: TNM staging, preoperative CEA and CD44v6 were independent prognostic factors for rectal cancer patients with total mesorectal excision. Postoperative chemotherapy may be only used together with radiotherapy for rectal cancer patients.
- Citation: Peng JJ, Cai SJ, Lu HF, Cai GX, Lian P, Guan ZQ, Wang MH, Xu Y. Predicting prognosis of rectal cancer patients with total mesorectal excision using molecular markers. World J Gastroenterol 2007; 13(21): 3009-3015
- URL: https://www.wjgnet.com/1007-9327/full/v13/i21/3009.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i21.3009
Colorectal cancer is the third leading cause of cancer death in both males and females. Approximately 35% of colorectal cancers are located in the rectum of patients from Western countries. In China the proportion reached approximately 50%.
New advances such as the standardized surgical technique total mesorectal excision (TME), preoprative or post-oprative radiotherapy and adjuvant chemoradiotherapy have reduced the previously high local recurrence rate and improved overall survival time in rectal cancer patients. Despite these advances, about 40% of patients still die from local or distant recurrence. Hence, new prognostic markers are required to help predict the patients who would benefit from adjuvant treatment.
The knowledge regarding the molecular biology of colorectal cancer has facilitated the study of molecular markers in patients with colorectal cancer. Several tumor associated proteins including p53, p21, p27, cyclin D1, PCNA, CD44, Ki67 may be relevant prognostic markers in rectal cancer. These markers were widely studied in many cancers including colorectal cancer, but the results related to prognosis and implications in colorectal cancer remain controversial, especially in rectal cancer. No single molecular marker has been demonstrated to provide consistent prognostic information yet.
Immunohistochemical (IHC) technique, which is easy, stable with experienced pathologists, and fast with commercially available antibody, is widely used in studies for molecular markers. In this study, the protein expression of p53, p21, PCNA and CD44 was examined with immunohistochemical technique to evaluate their prognostic value in rectal cancer patients undergoing curative total mesorectal excision (TME).
A total of 343 rectal cancer patients, who underwent total mesorectal excision in Cancer Hospital of Fudan University from January 1999 to June 2004, were collected retrospectively. The median follow-up time is 44 mo, ranging from 1-90 mo. Twenty-one cases (6.5%) who were lost at the beginning of the surveillance were excluded. Sixty-three patients with simultaneous distant metastases or lesions invading other organs (e.g. bladder, vesicle, prostate, posterior of vagina or urethra) were excluded in this study. All the surgeries were performed by experienced colorectal surgeons. Lateral lymphadenectomy was not performed in our series. A total of 259 patients were available after the screening.
The basic clinicopathological information is presented in Table 1. All cases were histologically confirmed adenocarcinoma and reviewed by two pathologists.
| Characteristics | Cases (%) |
| Gender | |
| Male | 146 (56.4) |
| Female | 113 (43.6) |
| Age (yr) | |
| Range | 18-80 |
| Median | 56 |
| Tumor location | |
| > 10 cm1 | 62 (23.9) |
| 7-10 cm1 | 115 (44.4) |
| 5-7 cm1 | 82 (31.7) |
| Mean Max diameter (cm) | 4.68 |
| Pathology | |
| Adenocarcinoma | 236 (91.1) |
| Mucinous aden3 | 18 (6.9) |
| Signet ring ca3 | 5 (2) |
| T stage | |
| T1 | 18 (7.0) |
| T2 | 83 (32.0) |
| T3 | 85 (32.8) |
| T4 | 73 (28.2) |
| N stage | |
| N0 | 147 (56.8) |
| N1 | 62 (23.9) |
| N2 | 50 (19.3) |
| TNM stage (AJCC/UICC) | |
| I | 80 (30.9) |
| II | 67 (25.9) |
| III | 112 (43.2) |
| Lymphovascular invasion | |
| Yes | 33 (12.7) |
| No | 226 (87.3) |
| Neural invasion | |
| Yes | 21 (8.1) |
| No | 238 (91.9) |
| Pre-operative CEA2 | |
| Positive | 48 (18.5) |
| Negative | 211 (81.5) |
| Adjuvant therapy | |
| S | 167 (64.5) |
| S + C | 92 (35.5) |
Adjuvant radiation was not routinely given to stage II or stage III patients with optimal total mesorectal excision with R0 resection before 2005 in our hospital. Only patients with T4 tumors below peritoneal reflex, which invaded other organs (bladder, prostate, vesicle, vagina, etc.) would receive postoperative radiotherapy or chemoradiotherapy. Chemotherapy with 5-Fu based regimenswas given to a part of patients with stage II or stage III disease and prospective observation was carried out to find out its effect in rectal cancer. None of the patients had received preoperative radiotherapy or chemoradiotherapy.
Two hundred and fifty-nine formalin fixed paraffin embedded tumor specimens were obtained at the department of pathology in the same hospital. These specimens were cut into 4 μm slides, dewaxed with dimethyl benzene and dehydrated in graded acetone. Tissues previously shown to express the antigen of interest were considered positive controls (i.e. colonic adenocarcinoma for p53, CD44v6, breast carcinoma for p21, normal colon for PCNA), and the primary antibody was replaced by TBS in the negative controls. A minimum of eight sections were examined per case, in which every two slides were used for a single marker.
All the 259 colorectal cancer specimens were collected and specific biological markers were analysed with immunohistochemical procedure, using the enVision two-step visualization technique (DAKO) which was described by Ulrike Kämmerer[1] and Schwandner[2]. The monoclonal antibodies, including anti-p53 (Clone DO-7,code no. M7001, DAKO, dilution, 1/50), anti-p21ras (Clone: NCC-RAS-001,code no. M0637, DAKO, dilution, 1/100), anti-PCNA (Clone PC 10,code no. M 0879,DAKO,dilution 1/300), and anti-CD44 variant 6 (Clone VFF-7,code no. M0130, Antibody Diagnostica,dilution 1/50) were used for immunohistochemical examination.
Immunostained tumor sections were analysed by two experienced pathologists without the knowledge of clinicopathological data. Sections immunostained for p53 and p21 were scored semi-quantitaitvely by scanning the entire section to estimate the percentage of tumor cell nuclear staining, and CD44v6 expression was estimated by the percentage of tumor cell membrane staining. The PCNA staining was expressed as a labeling index (LI) defining the positive nuclei of all the nuclei counted. The median value for the PCNA LI in this tumor series(59.5%,)was used as a cut-off point and tumors were classsified as either less than or greater than the median value.
For statistical analysis, p53 and p21 levels were considered to be positive if over 10% of cancer cells were nuclear immunoreactive; and CD44v6 was defined positive if over 10% of cancer cells were membrane immunoreactive.
Association between these proteins and clinicopathological data, and the univariate analysis between these data and prognosis were both performed by Chi-square test. The overall survival, local recurrence and metastasis rates were calculated using life tables. The multivariate analysis of these proteins and clinicopathological data was made using Cox regression. Significance levels were set at P < 0.05.
All patients were followedup every 3 to 6 mo at the Colorectal Cancer Center after surgery by their operative team. Follow-up included a full history and physical examinations including digital rectal examination (DRE) at each session. Chest X-ray, CT or ultrasound of abdomen, and lab tests were performed every 6 mo. And colonoscopy was performed every year for the first three years and then every 2 years. All surviving patients were asked to return to the Colorectal Cancer Center for follow-up for the purpose of this study.
Forty-eight patients exhibited elevated serum CEA levels. The disease stage, lymphnode metastastic status, lymvascular invasion, neural invasion, histopathology and tumor differentiation were not associated with CEA levels.
Among 179 stage II or stage III patients including 33 in stage II (49.3%) and 59 in stage III (52.7%), 92 (51.4%) received 5-Fu based adjuvant chemotherapy,. The detailed clincopathological information for these patientsis presented in Table 2.
| Adjuvant chemotherapy(n = 179) | P | |||||
| No | Yes | |||||
| Cases | % | Cases | % | |||
| N staging | N0 | 114 | 68.2 | 33 | 35.9 | < 0.05 |
| N1 | 33 | 19.8 | 29 | 31.5 | ||
| N2 | 20 | 12 | 30 | 32.4 | ||
| T staging | T1-2 | 90 | 53.9 | 11 | 12 | < 0.05 |
| T3 | 38 | 22.7 | 47 | 51 | ||
| T4 | 39 | 23.4 | 34 | 37 | ||
| Differentiation | High-Medium | 145 | 86.8 | 77 | 83.7 | > 0.05 |
| Low | 22 | 13.2 | 15 | 16.3 | ||
| Lymphovascular invasion | None | 19 | 11.4 | 14 | 15.6 | > 0.05 |
| Yes | 148 | 88.6 | 78 | 84.4 | ||
| Neural invasion | None | 14 | 8.4 | 7 | 7.6 | > 0.05 |
| Yes | 154 | 91.6 | 85 | 92.4 | ||
Of the 259 rectal carcinomas with anterior resection, 45.6% were p53 postitive (n = 118), 80.7% were p21 positive (n = 209), 49.8% were CD44v6 positive (n = 129), and 61.4% were PCNA positive (n = 159). There was no positive association among these four protein expressions.
The association between these markers and clinicopathological variables were analysed using Chi-square test, including tumor location, histopathological type, TNM staging, invasion depth, lymph node metastasis, neural invasion, lymphovascular invasion and preoperative CEA level. There was no significant difference in the distribution of these proteins and different clinicopathological variables, either (Table 3). But p21 expression was found to have significant association with histopathological type (P = 0.067) and invasion depth (P = 0.052).
| Characteristics | P53 (n) | P21 (n) | PCNA (n) | CD44 (n) | |||||||||
| + | - | P | + | - | P | + | - | P | + | - | P | ||
| Tumor location | High | 29 | 33 | NS | 53 | 9 | NS | 36 | 26 | NS | 32 | 30 | NS |
| Median | 53 | 62 | 93 | 22 | 70 | 45 | 63 | 52 | |||||
| Low | 36 | 46 | 63 | 19 | 53 | 29 | 34 | 48 | |||||
| Pathology | Adeno. | 109 | 127 | NS | 191 | 45 | < 0.05 | 144 | 92 | NS | 117 | 119 | NS |
| Muci | 8 | 10 | 16 | 2 | 13 | 5 | 11 | 7 | |||||
| Signet | 1 | 4 | 2 | 3 | 2 | 3 | 1 | 4 | |||||
| TNM stage | I | 34 | 46 | NS | 59 | 21 | NS | 47 | 33 | NS | 37 | 43 | NS |
| II | 30 | 37 | 58 | 9 | 42 | 25 | 33 | 34 | |||||
| III | 54 | 58 | 92 | 20 | 70 | 42 | 59 | 53 | |||||
| Invasion depth | T1-2 | 46 | 55 | NS | 74 | 27 | 0.052 | 63 | 38 | NS | 46 | 55 | NS |
| T3 | 36 | 49 | 72 | 13 | 55 | 30 | 43 | 42 | |||||
| T4 | 36 | 37 | 63 | 10 | 41 | 32 | 40 | 33 | |||||
| Lymphnode meta. | N0 | 64 | 83 | NS | 117 | 30 | NS | 89 | 58 | NS | 70 | 77 | NS |
| N1-2 | 54 | 58 | 92 | 20 | 70 | 42 | 59 | 53 | |||||
| Lymph-vascular invasion | + | 17 | 16 | NS | 25 | 8 | NS | 21 | 12 | NS | 19 | 14 | NS |
| - | 101 | 125 | 184 | 42 | 138 | 88 | 110 | 116 | |||||
| Neural invasion | + | 11 | 10 | NS | 18 | 3 | NS | 15 | 6 | NS | 11 | 10 | NS |
| - | 107 | 131 | 191 | 47 | 44 | 94 | 118 | 120 | |||||
| Preoperative CEA | + | 18 | 30 | NS | 41 | 7 | NS | 30 | 18 | NS | 109 | 102 | NS |
| - | 100 | 111 | 168 | 42 | 129 | 82 | 20 | 28 | |||||
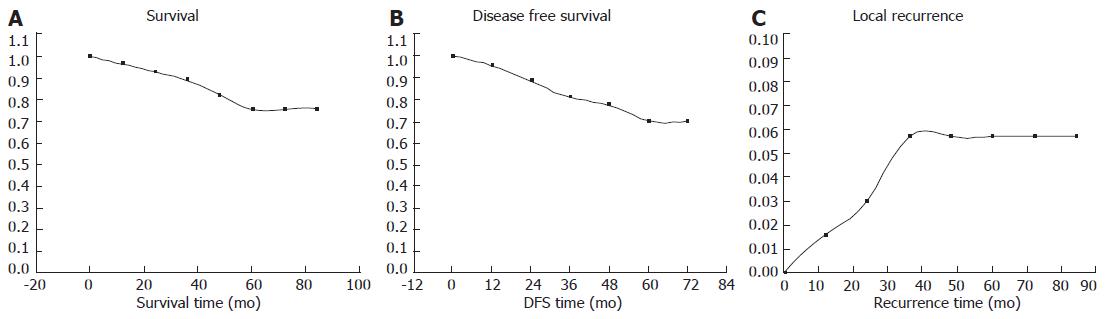
The median follow-up was 44 mo (range 1-90 mo). Thirty-three patients (12.7%) were dead due to tumor progression. Eleven patients (4.24%) had local recurrence, and 35 patients (13.5%) had distant metastases. The outcome of the patients is shown in Figure 1. Our five-year actual survival rate was 75.43% (Figure 1A), and disease free survival rate was 70.32% (Figure 1B). Overall local recurrence rate was 6.73% (Figure 1C).
In stage II and stage III locally advanced rectal cancer, our 5-year survival rate was 66.9%, disease free survival rate was 61.1%, and overall local recurrence rate was 8.9%.
Univariate analysis using Chi-square test as a screening method revealed that possible overall survival related risk factors were histopathological type, TNM staging, invasion depth, lymphnode metastasis, preoperative CEA and CD44v6 levels; possible disease free survival related risk factors were gender, histopathological type, TNM staging, invasion depth, lymph node metastasis and CD44v6 and preoperative CEA levels; possible local recurrence related risk factors were histopathological type, TNM staging, lymphnode metastasis; and possible metastasis related risk factors were TNM staging, invasion depth, lymphnode metastasis and preoperative CEA level (Table 4).
| n | Survival | DFS | Local recurrence | Metastasis | |||||
| % | P | % | P | % | P | % | P | ||
| Gender | |||||||||
| Male | 146 | 85.6 | NS | 78.1 | < 0.05 | 5.5 | NS | 16.4 | NS |
| Female | 113 | 89.4 | 87.6 | 2.7 | 9.7 | ||||
| Tumor location | NS | NS | NS | ||||||
| High | 62 | 88.7 | 85.5 | 3.2 | 12.9 | ||||
| Medium | 115 | 87.8 | 83.5 | 6.1 | 10.4 | NS | |||
| Low | 82 | 85.4 | 80.5 | 2.4 | 18.3 | ||||
| Pathology | < 0.05 | < 0.05 | < 0.05 | ||||||
| Adenocarcinoma | 236 | 87.7 | 83.9 | 3.8 | 13.1 | ||||
| Mucinous cancer | 18 | 94.4 | 83.3 | 5.6 | 11.1 | NS | |||
| Signet ring cancer | 5 | 40 | 40 | 20 | 40 | ||||
| Differentiation | |||||||||
| High-medium | 222 | 87.4 | NS | 82.9 | NS | 4.1 | NS | 13.1 | NS |
| Low | 37 | 86.5 | 78.4 | 5.4 | 16.2 | ||||
| TNM staging | < 0.05 | < 0.05 | < 0.05 | ||||||
| I | 80 | 95 | 92.5 | 1.3 | 6.3 | ||||
| II | 67 | 92.5 | 89.6 | 1.5 | 9.0 | < 0.05 | |||
| III | 112 | 78.6 | 70.3 | 8 | 21.4 | ||||
| Invasion depth | < 0.05 | <0.05 | NS | ||||||
| T1-2 | 101 | 95.0 | 91.1 | 2 | 6.9 | ||||
| T3 | 85 | 83.5 | 78.8 | 4.7 | 16.5 | < 0.05 | |||
| T4 | 73 | 80.8 | 75.3 | 6.8 | 19.2 | ||||
| N staging | < 0.05 | < 0.05 | < 0.05 | ||||||
| N0 | 147 | 93.9 | 91.2 | 1.4 | 7.5 | < 0.05 | |||
| N1-2 | 112 | 78.6 | 72.3 | 8.0 | 21.4 | ||||
| Lymphvascular invasion | NS | NS | NS | ||||||
| + | 33 | 81.8 | 78.8 | 6.1 | 15.2 | ||||
| - | 226 | 88.1 | 83.6 | 4 | 13.3 | NS | |||
| Neural invasion | NS | NS | |||||||
| + | 21 | 85.7 | NS | 85.7 | 4.8 | 14.3 | |||
| - | 238 | 87.4 | 82.8 | 4.2 | 13.4 | NS | |||
| Preoperative CEA | 0.06 | < 0.05 | NS | ||||||
| + | 48 | 20.8 | 31.3 | 6.3 | 25 | ||||
| - | 211 | 10.9 | 14.7 | 3.8 | 10.9 | < 0.05 | |||
| P53 | NS | NS | |||||||
| + | 118 | 84.4 | 80.9 | 6.4 | 0.06 | 13.5 | NS | ||
| - | 141 | 90.7 | 85.6 | 1.7 | 13.6 | ||||
| P21 | NS | NS | NS | ||||||
| + | 209 | 87.6 | 81.8 | 4.3 | 13.9 | NS | |||
| - | 50 | 86 | 84 | 4.0 | 12.0 | ||||
| PCNA | NS | NS | NS | 12.6 | |||||
| + | 159 | 89.3 | 84.3 | 3.1 | 15.0 | NS | |||
| - | 100 | 84.0 | 81.0 | 6.0 | |||||
| CD44 | < 0.05 | < 0.05 | NS | ||||||
| + | 129 | 82.9 | 76.7 | 6.2 | 17.1 | 0.97 | |||
| - | 130 | 91.5 | 87.8 | 2.3 | 10.0 | ||||
In all the 179 stage II or stage III patients, adjuvant chemotherapy had negative significant association with overall metastasis and disease free survival. But in stratification for each stage, adjuvant chemotherapy did not have any significance with local recurrence, overall metastasis, disease free survival and overall survival. One reason is that patients with more progressive disease were morelikely to receive adjuvant chemotherapy. For multivariate analysis, these possible risk factors screened in 259 rectal cancer patients by univariate analysis were included in Cox regression model. TNM staging, preoperative CEA, and CD44v6 level were independent risk factors predicting overall survival or disease free survival. The hazard ratio of peroperative CEA was 2.65 (95% CI 1.4-5) and 3.10 (95% CI 1.37-6.54) for disease free survival, and overall survival, respectively. The hazard ratio of CD44v6 was 1.93 (95% CI 1.04-3.61) and 2.21 (95% CI 1.01-4.88). TNM staging was the only risk factor predicting local recurrence.
In 179 stage II or stage III patients, we added the chemotherapy variable to Cox regression model, the results showed that adjuvant chemotherapy did not improve the overall survival, disease free survival or local recurrence in stage II or III patients.
The surgical management of primary rectal cancer presents unique problems for the surgeon basedlargely on the anatomic constraints of the pelvis. For most of the tumors over 5 cm above the anal verge, anterior resection was increasingly performed in recent years, occupying about 80%-90% of all rectal cancer surgeries in large centers. However, the local and distant recurrence was still challenging. The importance of mesorectum in rectal cancer surgery has been widely recognized. By total mesorectal excision, Heald et al[3] and Enker et al[4] had reported a lower local recurrence and improved the DFS and overall survival of the patients. In our series, overall local recurrence rate was 6.73%, disease free survival and overall survial were 70.32% and 75.43%, respectively, which was consistent with other studies about TME.
Many factors have been studied in predicting the outcome of the patients with rectal cancer who underwent total mesorectal excision. Whereas the use of clinical and histologic parameters for the determination of prognosis and treatment strategies for patients with rectal cancer is still of great value, they may be distressingly inaccurate in many clinical situations, especially in patients with stage II-III disease, which may need post- or pre-operative treatment. This may be attributed, at least in part, to differences in the biological behavior of tumors that are determined by altered molecular regulatory mechanisms. Thus, the characterization of molecular changes in colorectal cancer in recent years has been the focus of great interest for both researchers and clinicians, because it may lead to the identification of new prognostic markers more closely resembling the biological nature of the disease. Among the various alterations in gene and protein expression in colorectal cancers, cell-cycle control related genes and proteins (including p53, p21 and PCNA) and cell adhension protein CD44 were widely elucidated in many studies, but the prognostic values were still confusing, and very few studies exclusively focused on rectal cancer patients with anterior resection. In our series, we analyzed the prognostic effect of clinical variables and immnohistochemical markers. TNM staging, preoperative CEA and CD44v6 levels were recognized as prognostic factors predicting the disease free survival and overall survival.
Serum CEA level is a common preoperative and follow-up marker in colorectal carcinoma patients. Adenocarcinomas overexpress CEA, which may facilitate metastasis of colorectal carcinoma. Elevated preoperative serum levels are associated with high rates of recurrence and cancer mortality, and it should not be discarded in the current array of prognostic factors. Granell et al[5] studied preoperative CEA level and p53 expression in 134 colorectal cancer patients, and found patients with elevated preoperative CEA level were at significant high risk of local recurrence in two years after surgery, whose hazard ratio was 3.26. In our series, preoperative CEA level was an independent prognositic factorin predicting DFS and overall survival, the hazard ratio was 2.65 and 3.10, respectively. Our results suggested that preoperative CEA, like postoperative CEA, may be also a useful prognostic marker for rectal cancer patients.
The expression of specific cell adhesion molecule CD44 splice variants has been shown to be associated with metastasis and poor prognosis in certain human malignancies, such as breast cancer and colorectal cancer, especially the CD44 variant 6 (CD44v6)[6]. In most of these studies, increased levels of CD44 and/or different patterns of splice variants were found in tumors in comparison with their normal counterparts[7,8]. The studies addressing the relationship between CD44 expression at the protein level and clinicopathological variables, such as tumour grade and stage, have not been uniform. Ishida examined CD44v6 expression in 62 colorectal cancer patients, and the result showed CD44v6 has no correlation with gross type, histology, lymph node involvement, and clinical stage[9]. Bhatavdekar et al[10] examined CD44 in 98 Duke’s B and C colorectal adenocarcinomas with IHC, and they also found a significantly reduced relape-free survival in patients with positive CD44. Similary, Yamagnchi et al[11] have shown that CD44 is an independent prognostic factor in multivariate analysis. In our study, we did not find any significant association between CD44v6 and clinicopathological parameters either. But in multivariate analysis, we found CD44v6 was the independent biological prognostic marker for disease free survival and overall survival, and the hazard ratio was 1.93 and 2.21, respectively, suggesting CD44v6 is a valuable molecular marker for rectal cancer prognosis.
P53 was studied in colorectal cancer, but the results of IHC p53 rectal tumor status have been inconsistent. Hilska et al[12] studied 363 colorectal cancer patients, including 124 with rectal cancers from Duke’s stage A to D. The author s used different cut-off values for defining p53 positive, but none of them showed any significance for survival in all colorectal cancer groups. Morgan et al[13] studied 171 patients with curative resection of rectal cancer. By immunohistochemical assay for p53 and DCC expression, they found p53 and DCC status of rectal cancers was not associated with other clinical or pathological variables, nor predictive outcomes. The cyclin inhibitors p21 negatively regulates the action of cyclin/CDK complexes, and prevents cell-cycle progression. Lebe et al examined IHC p53 p21 and p27 expression in 45 rectal adenocarcinomas, and found p53, p21 and p27 status was not significantly associated with local and distant recurrence. PCNA is an auxiliary factor essential for DNA polymerases activity and exists in a quaternary complex with CDK/cyclin/p21. PCNA is frequently used to measure the proliferative activity of tissues, which was widely studied to evaluate the response of chemotherapy and radiotherapy. PCNA was found associating with improved survival in advanced colorectal cancer by Paradiso et al[14]. but several studies discovered no significant association between PCNA expression and prognosis in colorectal cancer[15-17]. In our study, we did not find any association between the three markers and clinicopathological variables. The three markers had no significant prognostic effect for predicting DFS or overall survival, either.
The benefit of adjuvant chemotherapy was of great controversy in rectal cancer patients. The EORTC Radiotherapy Group Trial 22921 found in 253 patients with postoperative chemotherapy, adjuvant chemotherapy was of benefit for local control in T3-4 rectal cancer patients[18]. In that clinical trial, patients were all assigned to receive preoperative radiotherapy or chemoradiotherapy. And the adherance to postoperative chemotherapy was very poor, which made the results accepted. Our patients received adjuvant chemotherapy alone after curative total mesorectal excision. We found in patients with curative excised rectal cancer, postoperative chemotherapy did not improve patients’ local control of the tumor or survival. The results suggested that postoperative chemotherapy may only improve the local control by enhancing the effect of radiotherapy. We therefore, do not recommand postoperative chemotherapy for stage II or III patients without preoperative radiotherapy.
This has been coupled in several series with an improved cancer-specific survival directly attributed to the performance of TME itself[19]. The outcomes are favorable for strictly defined curatively excised rectal cancers with meticulous total mesorectal excision. TNM staging, preoperative CEA, and CD44v6 levels are recognized as independent prognostic facotrs for these patients. And postoperative chemotherapy is not recommanded for curative excised rectal cancer patients without preoperative radiotherapy.
We would like to thank Hong-Feng Lu from the Pathological Department of Shanghai Cancer Hospital, Fudan University for providing us the specimens and her assistance with the immunohistochemistry. The assistance of Dr. Jia-De Lu in National University Hospital, Singapore is also gratefully acknowledged.
S- Editor Liu Y L- Editor Ma JY E- Editor Lu W
| 1. | Kämmerer U, Kapp M, Gassel AM, Richter T, Tank C, Dietl J, Ruck P. A new rapid immunohistochemical staining technique using the EnVision antibody complex. J Histochem Cytochem. 2001;49:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Schwandner O, Bruch HP, Broll R. Prognostic significance of p21 and p27 protein, apoptosis, clinical and histologic factors in rectal cancer without lymph node metastases. Eur Surg Res. 2002;34:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998;133:894-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1037] [Cited by in RCA: 1056] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 4. | Enker WE, Thaler HT, Cranor ML, Polyak T. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg. 1995;181:335-346. [PubMed] |
| 5. | Diez M, Pollan M, Müguerza JM, Gaspar MJ, Duce AM, Alvarez MJ, Ratia T, Herñandez P, Ruiz A, Granell J. Time-dependency of the prognostic effect of carcinoembryonic antigen and p53 protein in colorectal adenocarcinoma. Cancer. 2000;88:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Liu YJ, Yan PS, Li J, Jia JF. Expression and significance of CD44s, CD44v6, and nm23 mRNA in human cancer. World J Gastroenterol. 2005;11:6601-6606. [PubMed] |
| 7. | Stauder R, Eisterer W, Thaler J, Günthert U. CD44 variant isoforms in non-Hodgkin's lymphoma: a new independent prognostic factor. Blood. 1995;85:2885-2899. [PubMed] |
| 8. | Herrlich P, Pals S, Ponta H. CD44 in colon cancer. Eur J Cancer. 1995;31A:1110-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Ishida T. Immunohistochemical expression of the CD44 variant 6 in colorectal adenocarcinoma. Surg Today. 2000;30:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Bhatavdekar JM, Patel DD, Chikhlikar PR, Shah NG, Vora HH, Ghosh N, Trivedi TI. Molecular markers are predictors of recurrence and survival in patients with Dukes B and Dukes C colorectal adenocarcinoma. Dis Colon Rectum. 2001;44:523-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Yamaguchi A, Urano T, Goi T, Saito M, Takeuchi K, Hirose K, Nakagawara G, Shiku H, Furukawa K. Expression of a CD44 variant containing exons 8 to 10 is a useful independent factor for the prediction of prognosis in colorectal cancer patients. J Clin Oncol. 1996;14:1122-1127. [PubMed] |
| 12. | Hilska M, Collan YU, O Laine VJ, Kössi J, Hirsimäki P, Laato M, Roberts PJ. The significance of tumor markers for proliferation and apoptosis in predicting survival in colorectal cancer. Dis Colon Rectum. 2005;48:2197-2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Morgan M, Koorey D, Painter D, Findlay M, Newland R, Chapuis P, Solomon M. p53 and DCC immunohistochemistry in curative rectal cancer surgery. Int J Colorectal Dis. 2003;18:188-195. [PubMed] |
| 14. | Paradiso A, Rabinovich M, Vallejo C, Machiavelli M, Romero A, Perez J, Lacava J, Cuevas MA, Rodriquez R, Leone B. p53 and PCNA expression in advanced colorectal cancer: response to chemotherapy and long-term prognosis. Int J Cancer. 1996;69:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Sun XF, Carstensen JM, Stål O, Zhang H, Nordenskjöld B. Proliferating cell nuclear antigen (PCNA) in relation to ras, c-erbB-2,p53, clinico-pathological variables and prognosis in colorectal adenocarcinoma. Int J Cancer. 1996;69:5-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Díez M, Camuñas J, Enríquez JM, González A, Torabuela E, Gutiérrez A, Ratia T, Mugüerza JM, Martín A, Ruiz A. A comparative study on the prognostic value of the nuclear expression of protein p53 vis-à-vis histopathology in colorectal cancer. An Med Interna. 1996;13:222-226. [PubMed] |
| 17. | Neoptolemos JP, Oates GD, Newbold KM, Robson AM, McConkey C, Powell J. Cyclin/proliferation cell nuclear antigen immunohistochemistry does not improve the prognostic power of Dukes' or Jass' classifications for colorectal cancer. Br J Surg. 1995;82:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 2041] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 19. | Dahlberg M, Glimelius B, Påhlman L. Changing strategy for rectal cancer is associated with improved outcome. Br J Surg. 1999;86:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 138] [Article Influence: 5.3] [Reference Citation Analysis (0)] |