INTRODUCTION
Islet transplantation is considered a potentially curative treatment for diabetes mellitus. However, the protocol depends on the sufficiently large amounts of graft islets, requiring two to four cadaveric pancreas. The native islet vascular network has been ruined during the isolation[1]. It is estimated that > 70% of islet mass becomes stably engrafted[2]. So apart from immune rejection, another critical limitation to islet transplantation is the rate and extent of islet revascularization[3]. In this study, we used rat vascular endothelial cells (VECs) transfected with VEGF165 to elevate the production of vascular endothelial growth factor (VEGF) in the site of islet transplantation, and then determined whether the elevated VEGF expression could stimulate the angiogenesis in the grafts. These results would provide proof-of-principle that local expression of angiogenic molecules may enhance islet revascularization, thereby improving the outcome of marginal islet transplantation with better glycemic control in diabetic rats.
MATERIALS AND METHODS
Animals and materials
Wistar rats (body weight, 150 ± 20 g) were purchased from Animal Center of China Medical University. Diabetic rats were induced by streptozocin (STZ, IP, 60 mg/kg), and diabetes mellitus was defined when the non-fasting blood glucose level was greater than 16.8 mmol/L on two consecutive measurement[4]. Then the diabetic recipients were randomly divided into three groups (n = 10 in each group). In the control group, the islets were transplanted under the capsule of the right kidney. In the VEC group, vascular endothelial cells (VECs) were transplanted together with the islets. In the VEGF group, VECs transfected with pIRES2-EGFP/VEGF165 plasmid were transplanted together with the islets.
pIRES2-EGFP/VEGF165 was propagated in permissive cells and purified by CsCl density gradient centrifugation as previously described[5]. The titer of pIRES2-EGFP/VEGF165 was 1.1 × 1011 plaque-forming unit (pfu)/mL.
Islet isolation and transplant
Islets were isolated and purified according to the modified Minnesota program[6]. Briefly, after intraductal infusion of 10-12 mL of cold Hank’s balanced solution containing 1.5 mg/mL type V collagenase (C9263, Sigma), the pancreas was surgically procured and digested at 37°C for 15-20 min. During the digestion, the pancreas was observed closely, and digestion was stopped by RPMI1640 containing 200 mL/L serum when the emulsion appeared. The islets were purified by discontinuous Ficoll density gradient (25%, 23%, 20.5% and 11%) centrifugation at 3000 r/min for 10 min at 4°C. The distinct islets were collected and washed, and finally the 300 IEQ islets free of acinar cells, vessels, lymph nodes and ducts, which were considered as marginal grafts, were used for transplantation.
For the islet transduction by vectors, aliquots of the islets were incubated with VEGF vector at a defined multiplicity of infection (MOI) in 2 mL of serum-free RPMI1640 medium at 37°C for 2 h. After washing with Hanks’ balanced salt solution, transduced islets were used for transplantation.
Detection of islet function
Blood glucose and insulin levels were evaluated every other day after operation. The intravenous glucose tolerance test (IVGTT) was performed 10 d after transplantation. Rats were fasted for 5 h and injected intravenously with 500 g/L dextrose solution at a dose of 0.5 g/kg body weight, as previously described[7]. Blood glucose levels were measured before and at 1, 5, 10, 15, 30, 60 and 90 min after glucose infusion.
Histological observation
Hematoxylin and eosin (H&E) and immunohistochemical staining of islet grafts were performed. Briefly, animals were killed 14 d after transplantation, and islet grafts were retrieved from individual animals. After fixing in 10% phosphate-buffered formalin overnight, islet grafts were embedded in paraffin. The paraffin-embedded islet grafts were cut into consecutive sections (4-μm thick), which were immunostained with anti-insulin-6, rat anti-CD31 and rabbit anti-VEGF165 antibodies, respectively. Microvessel density (MVD) was determined under light microscopy after sections were immunostained with anti-CD34 antibodies, as previously described[8]. Clusters of stained endothelial cells were counted as a single microvessel. MVD expressed as average number of three highest area identified within a single 200 × field.
Statistical analysis
Data were expressed as mean ± SE. Statistical analyses of data were performed by ANOVA. Unpaired ANOVA analysis of variance was used to compare between two different treatment groups. P value < 0.05 was considered statistically significant.
RESULTS
Graft islet function after transplantation
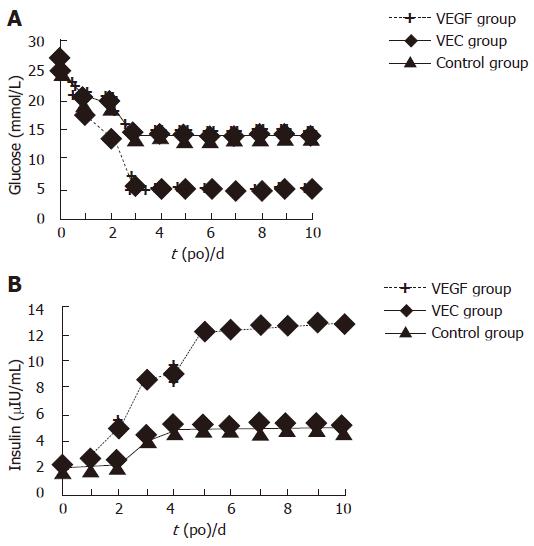
The blood glucose and plasma insulin levels in the diabetic recipients of the VEGF group restored to normal 3 d after transplantation. In contrast, diabetic rats receiving the same islets with or without normal VECs displayed moderate hyperglycemia (13-14 mmol/L), and insulin (? μIU/mL), without a significant difference between these two groups (Figure 1). We observed a significant difference in both the amplitude of blood glucose induction and kinetics of blood glucose decline between VEGF group and the other two groups, but no obvious difference between the control and VEC groups. In response to injection of a high dose of glucose, elevated blood glucose levels in the VEGF group were restored to a normal range within 90 min, whereas blood glucose levels in the control and VEC groups were increased to a significantly higher amplitude after glucose infusion and remained at the hyperglycemic level.
Figure 1 Glucose (A) and insulin (B) levels in the diabetic rats after transplantation.
Histological findings
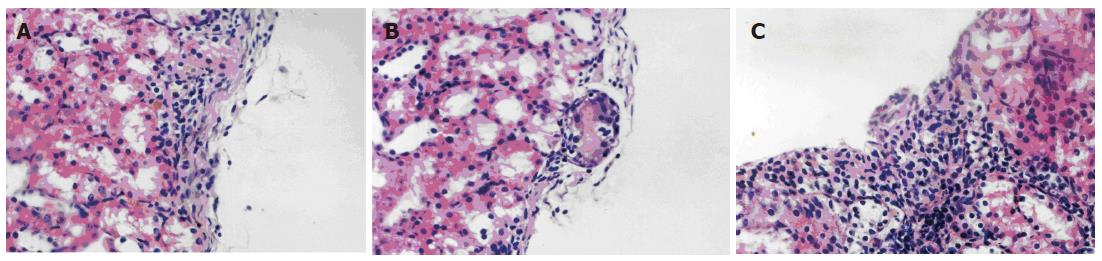
HE staining showed that a large amount of cell masses, which were different from the kidney native cells, were seen under the capsule of the kidney in the VEGF group. There were some vascular endothelial cells around and in the center of the masses. In the control and VEC groups, the similar cell masses were observed, but more fibrosis was seen in the center of the masses, where no vascular endothelial cells were seen (Figure 2).
Figure 2 HE staining of the graft islets under the kidney capsule (× 400).
A: Control group; B: VEC group; C: VEGF group.
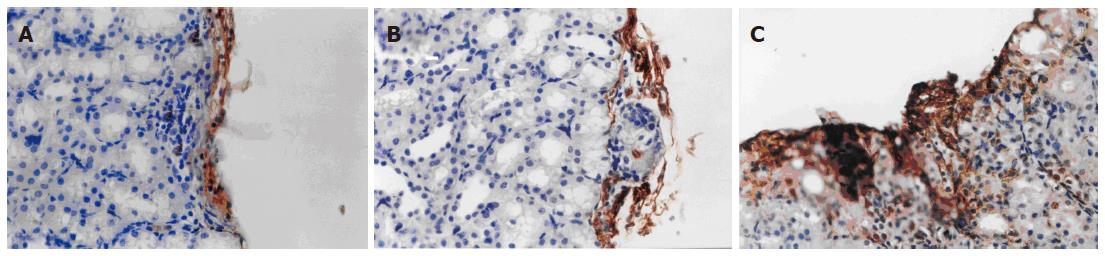
Immunohistochemistry showed a large amount of insulin-6-positive cells in cell masses of the VEGF group, whereas insulin-6-positive cells were seldom seen in the other two groups (Figure 3).
Figure 3 Immunohistochemical staining of insulin-6 in graft islets transplanted under the kidney capsule (× 400).
A: Control group; B: VEC group; C: VEGF group.
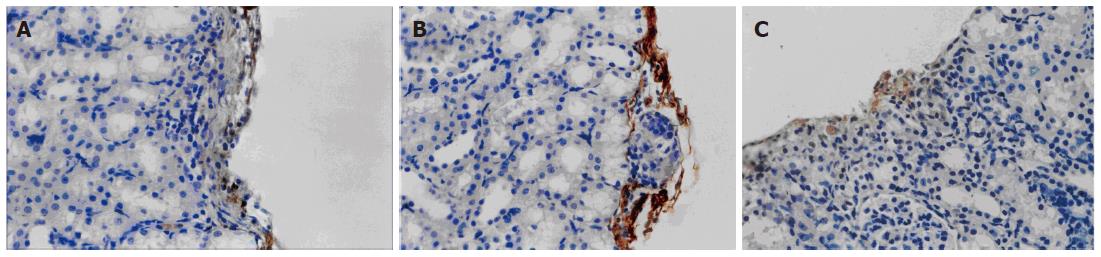
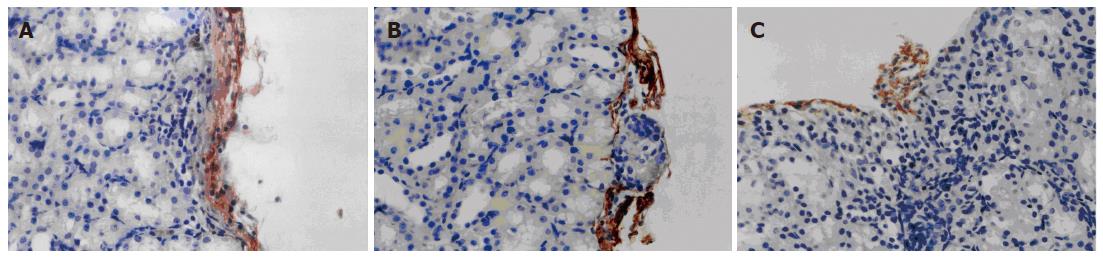
VEGF165 was expressed in the VEGF group but not in the control and VEC groups (Figure 4). As shown in Figure 5, a significant difference in the relative intensity of immunostaining for anti-CD34 antibody was observed between the VEGF and the other two groups, thereby indicating an increased degree of revascularization of transplanted islets in the VEGF group. MVD in the islet grafts was significantly higher in the VEGF group (74.3 ± 6.74) compared to the VEC group (11.43 ± 2.22) and control groups (10.9 ± 2.45) (P < 0.05).
Figure 4 Immunohistochemical staining of VEGF in graft islets transplanted under the kidney capsule (× 400).
A: Control group; B: VEC group; C: VEGF group.
Figure 5 Immunohistochemical staining of CD34 in graft islets transplanted under the kidney capsule (× 400).
A: Control group; B: VEC group; C: VEGF group.
DISCUSSION
Pancreatic islet transplantation has been proposed as a treatment for diabetic patients in order to restore physiological insulin secretion, to reduce hyper-/hypogly-cemia events and consequently to improve the quality of life. Successful islet transplantation depends on the infusion of sufficiently large quantities of islets, but more than 70% islets would become inactive in the early stage (7-14 d after transplantation)[9]. Many factors may contribute to islet death, including initial blood-mediated inflammation reaction (IBMIR), immunoattack, and ischemic injury due to insufficient blood supply[10-12]. Islet survival depends on the diffusion of oxygen and nutrients. Pancreatic islets are heavily vascularized, and they receive their blood supply from an afferent arteriole that branches into a glomerular-like network of microvessels, which forms a local intra-islet portal system through which blood flows from the central core of β cells to the non-β cell mantle. Islet capillaries are fenestrated, which may be important for the efficient release of secreted products into the bloodstream[13]. Moreover, β cells may require more oxygen than most other cell types, as suggested by the finding that islets constitute only 1% of the pancreatic mass but receive 10% of the pancreatic blood supply[14].
During the process of islet isolation, the circulation within the islets and the extra-islet vascularization are disrupted. Formation of new vessels may be too slow to allow survival of an optimal number of cells. Furuya et al[15] demonstrated that the native endothelial cell in the graft islets reduced gradually, and disappeared 5 d after the transplantation. New blood vessels develop over a period of about 14 d, or even 4 wk which is not fast enough to prevent the marked loss of islet cells occurring during the first few days after transplantation[16]. The mechanisms that regulate the revascularization of islets are poorly understood. Some studies[16] have suggested that the reduction of the endothelial cells from the donors may contribute to the poor revascularization. However, in our experiment, a large number of endothelial cells were transplanted together with the graft islets. We observed that in the early stage after transplantation, the process of revascularization was not changed and no vessels were formed although the endothelial cells survived.
It has been suggested that growth factors may be important in this process. VEGF is known to be one of the most important factors associated with angioge-nesis[17,18]. Therapeutic angiogenesis has been used for treating coronary and peripheral artery diseases by facilitating new vessel formation using plasmid or adenoviral vector-mediated VEGF gene delivery in a number of clinical trials[19-21]. VEGF may be a mediator in islets because it increases vascular permeability[22], which is important for the maintenance of normal endocrine function in highly vascularized organs and in devascularized islets[23]. VEGF may play a role in the maintenance of the islet capillary system[24] and initiate the revascularization of islets during transplantation[25]. VEGF expression is increased in the graft islets, but significantly reduced 2-3 d after transplantation and is not sufficient for revascularization[26,27]. Therefore, evaluation of local VEGF expression may provide a crucial clue for improving revascularization and survival of graft islets.
Our study aimed to investigate the effect of VEGF production on islet revascularization and function by transplanting the endothelial cells transducted by VEGF165 together with 300 IEQ islets, which is considered a marginal mass for diabetic rats. In the presence of islet impairment, revascularization appears to ensue irrespective of whether islets are transplanted intra-portally in the liver, retrogradely into the spleen, or under the kidney capsule. In our experiment, the islets were transplanted under the kidney capsule in order to facilitate histological observations. Unlike previous studies, we used endothelial cells, not the graft islets, as the “target cells” in our experiment. Endothelial cells take part in the revascularization, and are easy to obtain and be transducted. Our results validated the concept that elevated local production of VEGF helps facilitate islet revascularization, as reflected by the significantly increased CD34 immunostaining under the kidney capsule that was grafted with the VEGF vector-transduced endothelial cells.
The newly transplanted islets are hypoxic, causing islet cells to undergo apoptosis and/or necrosis, which contribute to the loss of functional β-cell mass after transplantation[28]. Our results also showed that the elevated VEGF expression contributed to the increased islet masses as reflected by H&E and immunohistochemical staining for insulin, which was associated with improved glycemic control in the diabetic recipient rats. Taken together, these results demonstrate that local VEGF production significantly enhances islet revascularization and improve glycemic control in STZ-induced diabetic rats. Our study may provide a novel strategy to accelerate islet revascularization and improve long-term survival of functional islet masses after transplantation.