Published online Jan 14, 2007. doi: 10.3748/wjg.v13.i2.316
Revised: October 8, 2006
Accepted: October 25, 2006
Published online: January 14, 2007
The early hypersensitivity reaction and late bone marrow depression are well-known side-effects of azathioprine, whereas interstitial pneumonia is a rare complication. A 40-year old male patient had been treated with azathioprine in consequence of extensive ulcerative colitis for 10 years. He then complained of 7 d of fever, cough and catarrhal signs, without symptoms of active colitis. Opportunistic infections were ruled out. The chest X-ray, CT and lung biopsy demonstrated the presence of interstitial inflammation. Azathioprine therapy was discontinued as a potential source of the pulmonary infiltrate. In response to steroid therapy, and intensive care, the pulmonary infiltrate gradually decreased within 4 wk. Three months later, his ulcerative colitis relapsed, and ileo-anal pouch surgery was performed. In cases of atypical pneumonia, without a proven infection, azathioprine-associated interstitial pneumonitis may be present, which heals after withdrawal of the drug.
- Citation: Nagy F, Molnar T, Makula E, Kiss I, Milassin P, Zollei E, Tiszlavicz L, Lonovics J. A case of interstitial pneumonitis in a patient with ulcerative colitis treated with azathioprine. World J Gastroenterol 2007; 13(2): 316-319
- URL: https://www.wjgnet.com/1007-9327/full/v13/i2/316.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i2.316
Azathioprine (Imuran® Glaxo-SmithKline) (AZA), one of the most commonly prescribed immunosuppressive drugs, is mainly administered in the treatment of immune-mediated diseases. AZA has been used to combat inflammatory bowel diseases (IBD) such as ulcerative colitis and Crohn’s disease since the 1960s[1]. It has been shown to reduce the number of lamina propria plasma cells, to alter the function of lymphocytes and natural killer cells and to exert an anti-inflammatory effect[2]. In IBD, azathioprine is used in both steroid-dependent and steroid-resistant cases. The clinical efficacy starts within 3-6 mo after the initiation of therapy. It is effective in more than half of the patients[3]. The early hypersensitivity reaction (nausea, fever, hepatitis and pancreatitis) and late bone marrow depression (leukopenia and macrocytosis) are relatively common side-effects[4], but interstitial pneumonitis is rare.
A 41-year old male patient with known ulcerative colitis since 1988 was referred to the Department of Medicine by his GP in June 2003 with symptoms and signs of relapse. His pancolitis had been treated with AZA (1.8 mg/kg per day) since 1993 because of frequent relapses. During the therapy only mild (1-2 blood-streaked stools) and rare (for 1-2 wk periods, once or twice yearly) relapses occurred. The patient was in good physical condition for his age and he was able to work. Six weeks before his admission, there was a severe relapse. He complained of anorexia, a weight loss (15 kg) and frequent bowel movements (10-12 times/d), accompanied with colicky pain and blood in the stools. Fever, dyspnoea and cardiac symptoms were not present. His treatment on admission was: 1 g olsalazine, 150 mg azathioprine, 12 mg methylprednisolone daily. Physical examination of the cardiorespiratory system was negative and diffuse abdominal tenderness was detected. His weight was 91 kg, his height was 182 cm, and his temperature was normal. The blood chemistry at the time of admission was as follows: ESR = 76 mm/h, serum sodium = 132 mmol/L, potassium = 3.7 mmol/L, haematocrit = 32%, hb = 111 g/L, TIBC = 55 μmol/L, white blood cell count = 4.400 G/L, and platelets = 368.000 G/L. Liver function tests were normal. Colonoscopy revealed severe active extensive ulcerative colitis involving the whole transversal colon. In response to parenteral corticosteroid treatment, his condition improved. He was discharged in good condition after 11 d on 48 mg/d oral methylprednisolone and 150 mg/d AZA.
After a remission period, which lasted throughout the summer, he presented to the Department of Medicine in September 2003 with complaints of weakness, weight loss, coughing and fever, the latter appearing every evening for a week. He did not cough up blood. He had 2-4 stools daily, without blood. He had no abdominal symptoms. His daily treatment on admission was 150 mg AZA, 16 mg methylprednisolone and doxycycline (prescribed by his GP). The results of physical examinations (cardiovascular and respiratory systems and abdomen) were normal. Septic fever was present. Blood chemistry values were as follows: ESR = 125 mm/h, haematocrit = 31%, hb = 10.7 g/L, white blood cell count = 4.100 G/L, platelets = 325.000 G/L, CRP = 351 mg/L, and uric acid = 471 μmol/L. The haemoculture was negative and procalcitonin was 0.07 ng/mL. The bacteriology (pharynx, sputum and urine) and virology (CMV, EBV, Coxackie and adenovirus) tests were negative.
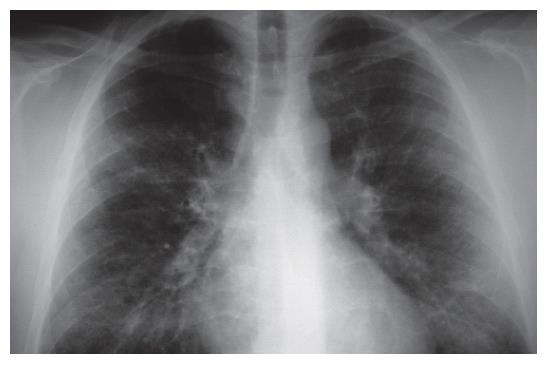
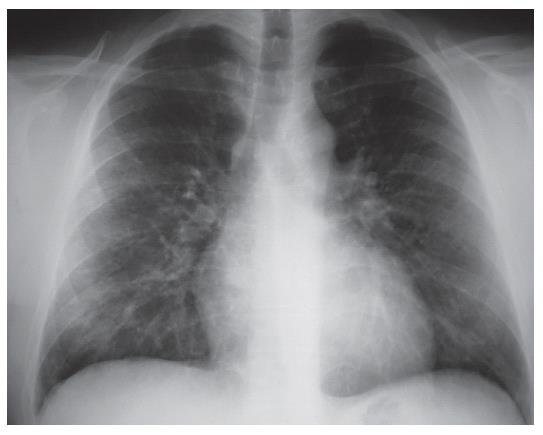
The chest X-ray (2003-09-09) revealed moderately increased interstitial shadowing with a ring-like consolidation in both lungs (predominantly in the right), without cardiac or aortic abnormalities. The follow-up chest X-ray revealed progression (Figure 1). Despite the discontinuation of AZA, progressive interstitial inflammation was detected in both lungs. The patient was treated with 1 g/d clarithromycin intravenously and 12 mg methylprednisolone orally. He was transferred to the intensive care unit because of the possibility of a severe opportunistic infection or an autoimmune disease. AZA-associated pneumonitis was also suspected.
His respiratory failure was treated in the intensive care unit. Dyspnoea occurred on minor exertion, the patient had orthostatic hypotension, and he was weak. On physical examination, the liver was palpable 2 cm beyond the costal margin. Except for dyspnoea nothing abnormal was detected. Blood chemistry showed ESR = 120 mm/h, haematocrit = 26%, hb = 90 g/L, white blood cell count = 2.970-3.200 G/L, platelets = 426.000 G/L, SGOT = 246 U/L, SGPT = 91 U/L, gamma GT = 600 U/L, LDH = 1125 U/L, and procalcitonin = 0.07 ng/L. Nasal oxygen supplementation was initiated, and a bronchial lavage sample was collected from the lower respiratory tract under anaesthesia. Arterial blood gas analyses displayed pH = 7.455, pCO2 = 33.0, pO2 = 50.9 mmHg, and sO2 = 86.2%. Two days later the corresponding values of pH, pCO2, pO2, and sO2 were: 7.394%, 41.7%, 75.1% and 94.0%, respectively. Neither typical nor atypical pathogens were detected in bacteriology samples. CMV PCR and Legionella IgM-IgG were also negative.
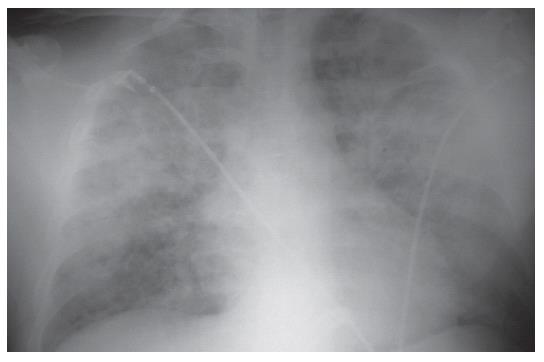
The follow-up chest X-ray examination (Figure 2), revealed a significant progression of the interstitial shadowing in both lungs. A palm-sized homogeneous consolidation was seen in the hilar area and marked interstitial shadowing in the remaining parts of the lungs. The radiological appearance was suggestive of acute respiratory distress syndrome (ARDS). Cardiac enlargement was demonstrated.
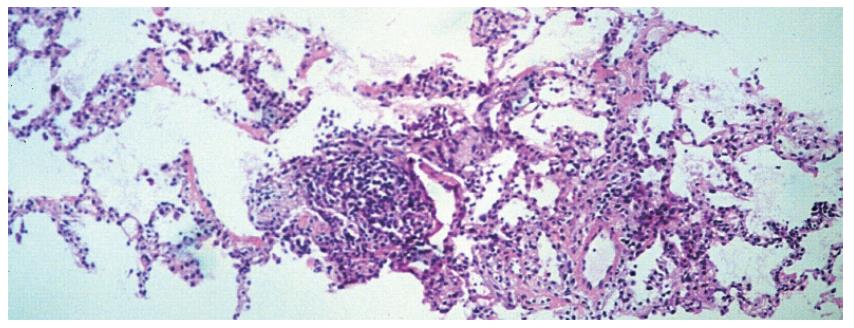
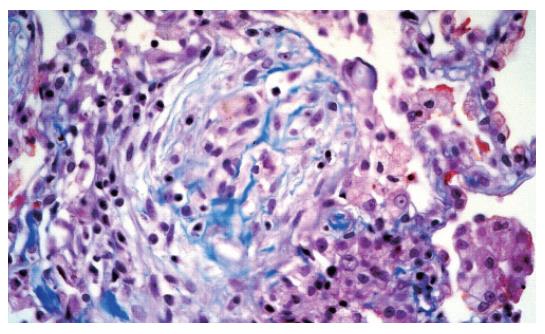
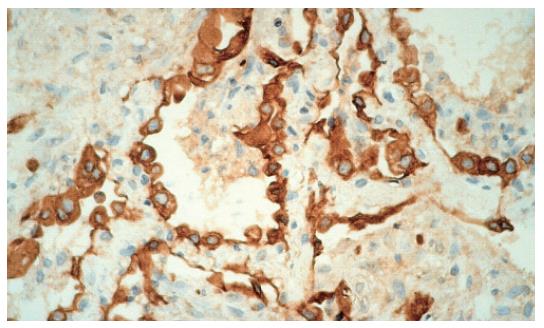
The chest CT examination revealed a 2-cm wide pleural effusion with increased alveolar/air-space shadowing bilaterally. The X-ray and CT examinations suggested ARDS. A needle biopsy was performed from the apex of the right lung. The histology showed intraluminal myxoid polyps (Masson bodies) in the alveoli (Figures 3,4,5). Vasculitis and Pneumocystis carinii infection were excluded. The results of the histology and immuno-histochemistry suggested a diagnosis of bronchiolitis obliterans organizing pneumonia (BOOP), but hypersensitivity pneumonitis was also regarded as a possibility. After sulphametoxasol/trimetroprim and methylprednisolone therapy, his condition gradually improved (Figure 6), and his liver and renal functions became normal. At the time of discharge, he was treated with 3 g sulphasalazine, 1 g sulphamethoxazole/trimethroprim and 8 mg methylprednisolone daily.
Three months later (2004-09-01) only mild dyspnoea occurred on exertion, but he had 8-10 liquid stools daily, occasionally with some blood on the surface of faeces. He had neither fever nor extraintestinal symptoms. The results of chest and heart examination were normal, but the sigma was tender. Corticosteroid therapy was restarted with 64 mg/d methylprednisolone. Blood chemistry showed serum Fe = 21.3 μmol/L, uric acid = 461 μmol/L, gamma GT = 121 U/L, cholesterol = 7.75 mmol/L, triglyceride = 2.3 mmol/L, white blood cell count = 7. 870 G/L and platelets = 223. 000 G/L. Pulmonary functions were VC = 110%, RV = 109%, spirometry FVC = 104%, FEV1 = 112%. The diffusion capacity values (DLCO = 74%, DLCO/VA = 60%) suggested a slightly decreased diffusion capacity. The chest X-ray revealed a further improvement of the lung volume. Interstitial shadowing was still present in the perihilar region of the lungs. Colonoscopy could only be carried out to 20 cm because of the pain and active colitis. The surface of mucosa was reddish, vulnerable and ulcerated, corresponding to active ulcerative colitis. In view of his previous history, proctocolectomy had to be considered. The first surgical intervention was carried out a month later and, after the ileal-pouch anal anastomosis (IPAA), the temporary anus prae was closed in August 2004. He had no complaints after the operation, produced 4-5 stools daily and was not on any medication.
In IBD patients, AZA treatment is worth starting if the patient is steroid-refractory (the effective dose does not lead to remission) or steroid dependent (discontinuation of the steroid causes a relapse)[5]. The first clinical results were inhomogeneous as concerns efficacy, because of the long time until the onset of action. Hawthorne et al[6] have proven the beneficial effect of the medication on the maintenance of remission in ulcerative colitis. In their study, seventy-nine patients who had been taking AZA for 6 mo were then divided into two groups. One of the groups continued to receive the therapy while placebo was administered to the patients in the other. The relapse rate was 36% in the AZA-treated group and 59% in the placebo group.
The mechanism of action of AZA is unknown. In IBD, the drug decreases the number of lamina propria plasma cells and alters the functions of lymphocytes and natural killer cells.
The therapeutic effect has been explained by the apoptosis-inducing behaviour of the drug. Tiede et al[7] considered that 6-thioGTP, a metabolite of AZA, could alter the apoptosis of T cells. The metabolite inhibits the activation of certain genes inside the cells, and induces apoptosis via a mitochondrial path.
Bedrossian et al[8] have noted bilateral lung infiltration with a reduced pO2 in 7 kidney-transplanted patients treated with AZA, which was not cured by antibiotic therapy. In their study, lung biopsy revealed an abnormal histology, which varied from diffuse alveolar damage to interstitial pneumonia and pulmonary fibrosis. No immune deposition, eosinophilia, vasculitis or granuloma could be detected, and the bacteriologyical findings were negative. When AZA was withdrawn from the treatment, the infiltration was significantly diminished in 2 patients who exhibited diffuse alveolar damage, but 4 patients diagnosed as interstitial pneumonitis, died of ARDS. The biopsies revealed hyaline membranes, intraalveolar oedema and damage to the alveolar epithelium in those who received low doses of AZA, and atypical epithelial hyperplasia, reorganization of the distal air spaces and fibrosis in the cases treated with high doses of AZA. Histologically the changes observed could not be distinguished from the characteristic changes caused by other pulmonary toxic drugs.
In our case, AZA treatment, initiated because of the common and serious relapses of extensive ulcerative colitis, ensured a good condition for almost 10 years. The patient was hospitalized because of a severe relapse in June 2003, but at that time no pulmonary abnormality was found. Remission was achieved with intravenous corticosteroid therapy. Three months later (2003-09-09), the patient presented with cough, catarrhal signs and pulmonary infiltration. We initially thought of concomitant infection, which may be expected during immunosuppressive treatment. In consequence of earlier therapy the pancolitis was in remission. After admission, the dose of AZA was reduced, and doxycycline was changed to chlarythromycin. However, despite his good general condition and the conventional treatment, the pulmonary infiltration rapidly deteriorated. Dyspnoea developed, which necessitated intensive care. Concomitant infection was not confirmed by the diagnostic examinations, and broad-spectrum antibiotics were proven ineffective. The lung biopsy results and the clinical features strongly suggested that he had AZA-associated interstitial pneumonia. Other causes of BOOP, such as eosinophilic pneumonia, vasculitis, irradiation, nitrofurantoin, gold, amiodarone and methotrexate, were excluded. In view of the results, AZA treatment was discontinued, oral corticosteroid medication was continued and the patient’s condition gradually improved. Three months later (December 2003), ulcerative colitis relapsed. In view of the clinical course of ulcerative colitis (frequent recurrences and AZA-induced interstitial pneumonitis), proctocolectomy was recommended. After surgery, the patient’s condition normalized without any further treatment.
In summary, in cases of atypical pneumonia without a proven opportunistic infection, AZA-associated interstitial pneumonitis may be present, which heals after withdrawal of the drug.
S- Editor Wang J L- Editor Wang XL E- Editor Liu WF
| 1. | Dubinsky MC. Azathioprine, 6-mercaptopurine in inflammatory bowel disease: pharmacology, efficacy, and safety. Clin Gastroenterol Hepatol. 2004;2:731-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 175] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Siegel CA, Sands BE. Review article: practical management of inflammatory bowel disease patients taking immunomodulators. Aliment Pharmacol Ther. 2005;22:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53 Suppl 5:V1-V16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 772] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 4. | Domènech E. Inflammatory bowel disease: current therapeutic options. Digestion. 2006;73 Suppl 1:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Nagy F. Conservative therapy of inflammatory bowel diseases. Orv Hetil. 2002;143:2763-2768. [PubMed] |
| 6. | Hawthorne AB, Logan RF, Hawkey CJ, Foster PN, Axon AT, Swarbrick ET, Scott BB, Lennard-Jones JE. Randomised controlled trial of azathioprine withdrawal in ulcerative colitis. BMJ. 1992;305:20-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 317] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, Lehr HA, Wirtz S, Becker C, Atreya R. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003;111:1133-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 573] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 8. | Bedrossian CW, Sussman J, Conklin RH, Kahan B. Azathioprine-associated interstitial pneumonitis. Am J Clin Pathol. 1984;82:148-154. [PubMed] |