Published online Jan 14, 2007. doi: 10.3748/wjg.v13.i2.306
Revised: July 25, 2006
Accepted: October 27, 2006
Published online: January 14, 2007
While cardiopulmonary symptoms are common in patients undergoing classical or, due to physical exercise, exertional heat stroke, the failure of other organs is a rarely described phenomenon. Here we present two cases of acute hepatic failure, one due to classic heat shock, while the other occurred while the patient was doing a marathon-type running. Both cases presented with very high transaminases and significantly elevated international normalized ratio (INR). No other causes for liver failure could be identified but physical exhaustion and hyperthermia.
- Citation: Weigand K, Riediger C, Stremmel W, Flechtenmacher C, Encke J. Are heat stroke and physical exhaustion underestimated causes of acute hepatic failure? World J Gastroenterol 2007; 13(2): 306-309
- URL: https://www.wjgnet.com/1007-9327/full/v13/i2/306.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i2.306
Heat stroke is a life-threatening condition that can be fatal if proper assessment and treatment are not initiated rapidely[1,2]. A variable degree of organ involvement is present in heat stroke[3]. At the beginning, there is heat exhaustion, characterized by nonspecific symptoms such as malaise, headache and nausea. Untreated this illness results in heat stroke, a serious disease possibly involving central nervous system dysfunction, rhabdomyolysis, arrhythmias, disseminated intravascular coagulation and hepatic failure, not uncommon followed by death[4].
The current model of heat stroke favors hyperthermia as trigger, while endotoxaemia drives the disease. However, the pathology is not fully understood[2]. In athletes undergoing intense training a variety of immune and gastrointestinal disturbances can occur. We here describe two completely different causes of heat stroke resulting in acute and severe liver failure as leading symptom.
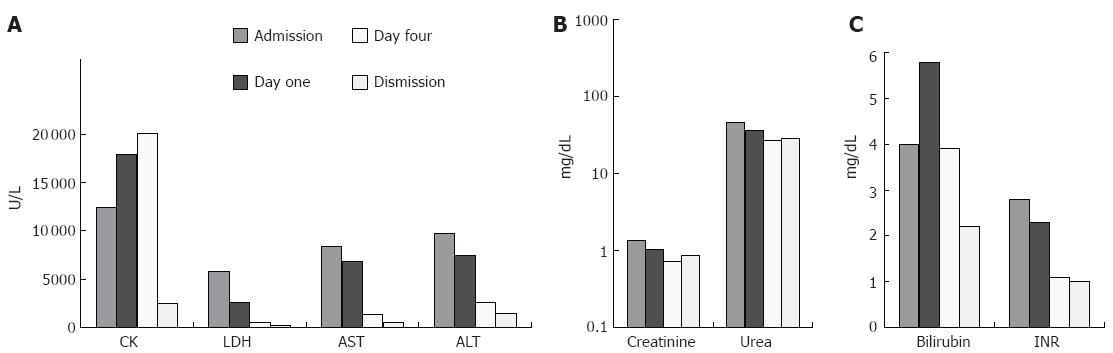
A 23-year old male was delivered to our intensive care unit (ICU) after he collapsed running a half-marathon. He had no former medical history and was in good physical shape. He took no medication and was negative for an obtained drug screening. By admission he felt fatigue and complained about nausea and vomiting. The laboratory results showed elevated levels of 12427 U/L creatinine kinase (CK) (normal < 145 U/L), 5821 U/L lactate dehydrogenase (LDH) (normal < 248 U/L), 8378 U/L aspartate aminotransferase (AST) (normal < 31 U/L), 9765 U/L alanine aminotransferase (ALT) (normal < 34 U/L), 40 mg/dL bilirubin (normal < 1.0 mg/dL) and 2.8 international normalized ratio (INR) (normal < 1.2) (Figure 1). The other basic laboratory parameters were within normal range. The patient was monitored and treated with intravenous fluid (5 to 6 liters per 24 h).
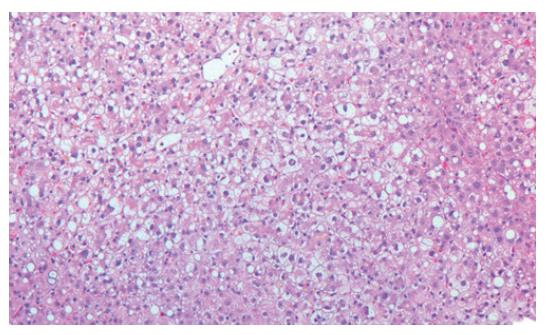
To exclude other causes for acute hepatic failure, virus serologies were obtained. Besides positive IgG of hepatitis A and B due to immunization, there were no serological findings for acute or chronic hepatitis A, B, C or human immunodeficiency virus (HIV). Also acute infection with cytomegaly virus (CMV), herpes simplex virus (HSV) or epstein-barr virus (EBV) was ruled out. The autoimmune antibodies (ANA, ANCA, SMA, LKM, mitochondrial antibodies) were also negative. In addition, protein electrophoresis gave no pathologic findings. Serum levels for alpha-1-antitrypsin, iron and transferrin were within normal range. Ferritin was raised to 29490 μg/L (normal 30-300 μg/L), probably due to destruction of hepatocytes. Because of lowered caeruloplasmin (serum) and copper (serum and 24-h urine) levels, a liver biopsy was performed. The histology showed intact architecture of the lobules. Portal fields showed no signs of fibrosis or inflammatory infiltration. Accentuated centrolobular necrosis and fatty degeneration of hepatocytes were seen. Additionally, focal bile inclusions were found. Altogether, the morphological picture did not fit acute Wilson’s disease, hemochromatosis or infection, but ischemic liver disease (Figure 2).
Abdominal ultrasound including duplex sonography, besides a slight hepatosplenomegaly, demonstrated no abnormal findings. To exclude a cardiac reason for the patient’s collapse, both a 24-h electrocardiogram and an echocardiogram showed normal heart function and structure.
The patient stayed in the ICU for 5 d, while the laboratory results were declining (Figure 1) (208 U/L LDH, 676 U/L AST, 1438 U/L ALT, 2.2 mg/dL bilirubin, 1.1 INR). Initially, the CK did rise to levels higher than 30 000 U/L, but on d 5 after admission it dropped to 4948 U/L. Renal function was normal at all times due to high fluid application and keeping the urine-pH above 7.5. On d 6, the patient was transferred from ICU to a regular ward in good health and mostly recompensated laboratory parameters for further monitoring. At follow-up one week later the patient was in good health and showed normal laboratory parameters.
A 46-year old male was delivered to our ICU after he had collapsed due to heat shock. His working place was below a roof window and it was a very hot day. In the after-noon, after working all day, he finally suffered from a seizure and was found cardiorespiratory stable but unconscious with his temperature of 42°C.
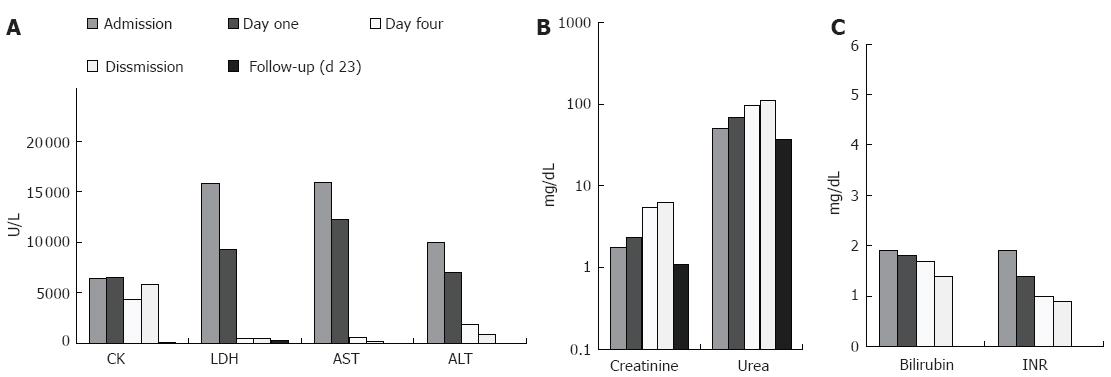
By admission laboratory findings demonstrated a clinical picture of acute hepatic failure with very high levels of transaminases (15 929 U/L AST, 10 050 U/L ALT), bilirubin (1.9 mg/dL) and malfunction in liver synthesis (1.9 INR) (Figure 3). Further laboratory parameters showed an elevated LDH (15 831 U/L), CK (6452 U/L), Troponin T (1.18 μg/L, normal < 0.03 μg/L), creatinine (1.78 mg/dL, normal < 1.3 mg/dL) and urea (50 mg/dL, normal < 45 mg/dL). All other basic parameters were within normal range.
Besides a seizure in 1989 due to an intra-cerebral bleeding, the patient had no past medical history. We started to treat him with intravenous fluid (5 to 6 liters per 24 h). Because of acute renal dysfunction he was treated two times with hemodialysis.
To exclude other reasons for acute hepatic failure he received further examinations. An abdominal ultrasound including duplex sonography showed no alterations. No pathologic serum markers for alpha1-antitrypsin, caeruloplasmin, copper, iron, ferritin, AFP or autoimmune antibodies (ANA, ds-DNA, ANCA, LKM, SMA, mitochondrial antibodies) were observed. Virus serologies were positive for HSV-IgG, VZV-IgG, EBV-IgG, but negative for IgM, as a parameter for acute infection. Hepatitis A was IgG positive due to immunization. All other infectious causes tested, like CMV, influenza A and B, hanta virus, hepatitis B and C, HIV and leptospirosis, were negative. Additionally, an obtained drug screening was negative.
The patient was further monitored in our ICU and received treatment of cooling and intravenous fluid. The patient regained consciousness within 36 h after therapy was started and the abnormal laboratory parameters excluding creatinine and urea, returned slowly to normal within a few days. Because of the elevated myocardial enzymes at admission, an echocardiogram and an electro cardiogram (ECG) were obtained. While the ECG was normal, the echocardiogram showed a very mild hypertrophic obstructive cardiomyopathy (HOCM), unknown so far, but a good right and left ventricular function and no contraction disorders of the myocardium. Also, the patient did not suffer from chest pain or dyspnoea at all times.
Six days after admission the patient had lowered laboratory parameters (Figure 3) (512 U/L LDH, 620 U/L AST, 1889 U/L ALT, 1.7 mg/dL bilirubin, 4376 U/L CK, 0.35 μg/L TNT, 1.0 INR), but still high urea and creatinine levels. He was transferred to a nephrology ward where he continued to get hemodialysis, until the kidney function was resolved. Twenty-three days later he presented again at the outpatient clinic with almost normalized laboratory parameters (Figure 3).
Described in this paper are two cases of acute hepatic failure due to physical exhaustion/heat shock. While the first patient had no other organ disorders, the second suffered from multi-organ failure including heart, kidneys and brain, and mainly liver malfunction.
It has been rarely reported that physical exhaustion can lead to hyperthermia, coma, rhabdomyolysis and organ failure[5,6]. Classic heat stroke however, is a more common disease with the life-threatening core body temperature higher than 40.5°C. Its associated clinical manifestations are exsiccosis, fatigue, nausea, vomiting, disorientation and coma[7]. Possible complications are cardiopulmonary dysfunction, acid-base or electrolyte disorders, as well as failure of other organs. Recently, a study demonstrated that 21 of 28 patients with heat stroke developed organ dysfunctions. Acute respiratory distress syndrome is the frequently encountered complication[8], while liver malfunction has not been reported. Our two cases indicate that liver failure may be more often than expected. Liver failure due to heat stroke begins with the same common symptoms, like nausea and exhaustion. When collapse, hyperthermia and multi-organ failure occur, it may be diagnosed[9], indicating that a rise in liver enzymes is an important predictive factor[8]. In our cases, liver failure was the leading symptom, causing cerebral disorders and renal dysfunction. Liver transplantation is the only possible treatment for severe hepatic damage. Recently, the first long-term follow-up of liver transplantation for hepatic failure due to heat stroke has been reported[10].
The recommended treatment for such severe cases is fluid application and rebalancing acid-base and electrolyte disorders as well as close monitoring, except for liver transplantation. Other treatments, like application of human umbilical cord blood cells as described in rats, to lower intracerebral changes due to heat stroke, remain speculative and under experiment at present[11].
The mechanism underlying liver failure in heath shock patients is not totally understood. An earlier study showed that systemic or intrahepatic circulatory disturbance as seen in disseminated intravascular coagulation (DIC), may be the cause[12]. In one case, a portal vein thrombosis has been found[9], which could result in liver and other organ failure. However, in our cases, the duplex sonography showed no big vessel thrombosis, whereas DIC could not be excluded. Although DIC could explain the raised liver enzymes in our second case, the cardiac problems due to HOCM contributing to the liver failure could not be ruled out. Nevertheless, we think that a cardiac reason is unlikely, because the right and left ventricular function of the patients was excellent at all times.
It has been reported that the intracranial pressure rises and cerebral ischemia occurs due to lower arterial pressure during heat stroke, resulting in lower blood flow and intracranial pO2[11]. It remains for further study if the same mechanism is responsible for liver damage. If so, this could result in a therapeutic application of vasoactive substances to increase intrahepatic blood flow in patients with severe liver damage after heat stroke.
Another review published in 2004 has discussed a possible function of immune response in organ failure of patients suffering from hyperthermia during physical exercise[13]. Since a reduced splanchnic blood flow which can result in gastrointestinal barrier dysfunction and increased permeability can be measured, endotoxin causing immune reactions could enter internal organs and drive organ damage. This may lead to the assumption that application of immune suppressive medication could be tested.
Besides liver malfunction, renal problems were the leading cause of liver failure in our cases. This is most likely explained by exsiccosis. Nevertheless, it is also possible that the high CK is an additionally cause of renal disorder.
In conclusion, patients with hepatic failure due to physical exhaustion or heat stroke should be closely monitored and severe problems like bleeding, hepatic coma and acute renal malfunction should be recognized as early as possible.
S- Editor Wang GP L- Editor Wang XL E- Editor Ma WH
| 1. | Casa DJ, Armstrong LE, Ganio MS, Yeargin SW. Exertional heat stroke in competitive athletes. Curr Sports Med Rep. 2005;4:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Lim CL, Mackinnon LT. The roles of exercise-induced immune system disturbances in the pathology of heat stroke: the dual pathway model of heat stroke. Sports Med. 2006;36:39-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Sucholeiki R. Heatstroke. Semin Neurol. 2005;25:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Glazer JL. Management of heatstroke and heat exhaustion. Am Fam Physician. 2005;71:2133-2140. [PubMed] |
| 5. | Lepape A, Sarron C, Grozel JM, Perdrix JP, Banssillon V. A severe form of heat stroke in a long-distance runner. Ann Fr Anesth Reanim. 1986;5:441-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Bruguera M. Liver and sports. Med Clin (Barc). 2004;122:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Yeo TP. Heat stroke: a comprehensive review. AACN Clin Issues. 2004;15:280-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Varghese GM, John G, Thomas K, Abraham OC, Mathai D. Predictors of multi-organ dysfunction in heatstroke. Emerg Med J. 2005;22:185-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Scobie BA. Gastrointestinal emergencies with marathon-type running: omental infarction with pancreatitis and liver failure with portal vein thrombosis. N Z Med J. 1998;111:211-212. [PubMed] |
| 10. | Takahashi K, Chin K, Ogawa K, Kasahara M, Sakaguchi T, Hasegawa S, Sumi K, Nakamura T, Tamaki A, Mishima M. Living donor liver transplantation with noninvasive ventilation for exertional heat stroke and severe rhabdomyolysis. Liver Transpl. 2005;11:570-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Chen SH, Chang FM, Tsai YC, Huang KF, Lin MT. Resuscitation from experimental heatstroke by transplantation of human umbilical cord blood cells. Crit Care Med. 2005;33:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Irie H, Mori W. Fatal hepatic necrosis after shock. Acta Pathol Jpn. 1986;36:363-374. [PubMed] |
| 13. | Lambert GP. Role of gastrointestinal permeability in exertional heatstroke. Exerc Sport Sci Rev. 2004;32:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |