INTRODUCTION
In 1984, Keim and co-workers reported the presence of a new protein in pancreatic juice of rats after induction of acute pancreatitis[1]. This secretory protein was absent in control rats but appeared early after induction of pancreatitis and remained over-expressed for the following 3-4 d. The protein was detected also in pancreas homogenate and in zymogen granules. Due to the relationship with the induction of pancreatitis, the protein was denominated “pancreatitis associated protein” or PAP. Four years later, Tachibana and colleagues described the peptide 23 as a protein from the rat pituitary gland, which synthesis was stimulated by the growth hormone-releasing hormone and inhibited by somatostatin[2] but its primary structure remained unresolved at the time. The sequence of PAP was deduced after cloning the corresponding mRNA from rat[3] and human pancreas[4]. Only four years later Katsumata and co-workers reported that in fact, peptide 23, identified in 1988, was identical to PAP[5]. Finally, Lasserre and colleagues found that the PAP mRNA was overexpressed in 7 of 29 hepatocellular carcinomas[6] and named the encoded protein HIP. Therefore, peptide 23, HIP and PAP are three names for the same protein. In this review we will call it PAP because it is the first name adopted for this protein.
In healthy pancreas, PAP is constitutively expressed in the α-cells of Langerhans islets[7]. By contrast, in the exocrine pancreas, PAP is only expressed when the acinar cells are harmed[4]. In fact, PAP expression is activated in pancreatic acinar cells in response to many injuries such as acute and chronic pancreatitis[4], hypoxia[8], toxins[9], diabetes[10], lipopolysaccharides[11], hypotransferrinaemia[12], and in the transplanted tissue[13]. However, its expression is not restricted to pancreatic tissue, and could be observed in several organs. This includes the intestine during chronic inflammatory diseases such as Crohn’s disease and ulcerative colitis[14,15] and in animal models of inflammatory bowel disease (IBD)[16]. PAP is also expressed in the brain tissue of Alzheimer patients[17,18], in the luminal epithelial cells of the uterus[19] and in a sub-population of developing motoneurons and, after peripheral injury, in sensory neurons and motoneurons[20,21]. Moreover, PAP mRNA expression was found activated in about 80% of the pancreatic adenocarcinomas of ductal origin and in 30% of mucinous cystadenomas[22]. The levels of PAP mRNA expression correlated with nodal invasion, presence of distant metastases and short survival. Also, in some cases peritumoral regions overexpressed PAP[23], indicating that both tumor and peritumoral cells contribute to the high PAP serum level observed in patients with pancreatic cancer. In the liver, PAP was found strongly activated in about 30% of the primary hepatocarcinomas, but the forced expression of PAP in this organ does not induce tumor development[24].
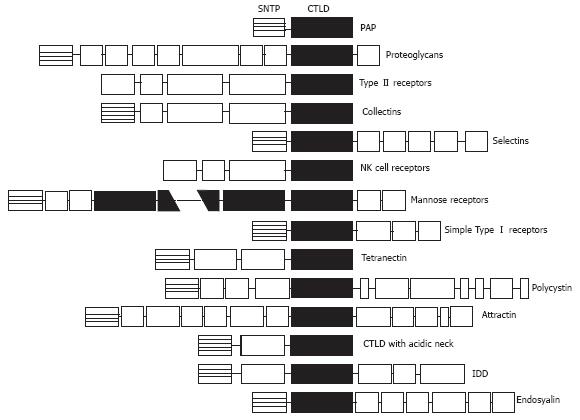
PAP IS A LECTIN-RELATED PROTEIN
Computer analysis of the PAP sequence suggests a structural relationship with lectins[3]. Identities between PAP and the other animal and human lectins range from 16% to 26%. Homologous domain includes the conserved amino acids of the consensus carbohydrate binding domain of the Ca2+-dependent lectins (C-type lectins) (Figure 1). However, initial attempts to characterize a carbohydrate binding activity, including erythrocyte agglutination or adsorption to affinity columns specific for different carbohydrates, failed[3]. The structural organization of the PAP gene reveals new clues to the evolutionary development of the lectin genes. The PAP coding sequence spans over six exons and the putative carbohydrate-recognition domain is encoded by exons IV, V and VI. This gene organization suggests that PAP belongs to a new group of lectins which have evolved from the same carbohydrate-recognition domain ancestral precursor through a different process[25]. It is interesting to note that PAP is the smallest protein reported among the C-type lectins. In fact, it comprises a single carbohydrate-recognition domain linked to a signal peptide[26] whereas other C-type lectins contain the sugar-binding consensus combined with a variety of other protein domains which confer specific functions of lectins. In contrast, PAP does not have additional functional domains.
Figure 1 Schematic protein domains in the different C-type lectins.
Sequences are aligned on the C-type lectin-like domain (CTLD). Note that PAP is the smallest protein member, containing only the CTLD linked to a short N-terminal peptide (SNTP).
PAP IN PANCREATIC DISEASES
Early after its discovery it becomes clear that acinar cells of the pancreas are the main source of PAP in pathological situations. Using an experimental model of acute pancreatitis in rat, Morisset and co-workers found the induction of PAP and its localization in zymogen granules[27]. Bodeker and co-workers described the pattern of PAP up-regulation in exocrine pancreas during the progression of the disease[28]. The profiles obtained by Northern blot analysis with pancreatic RNAs, Western blot in pancreatic protein extracts and immunodetection are equivalent to that observed in human disease and in animal models. The absence of PAP in healthy pancreas and its strong induction observed during the early phase of the disease suggest that PAP could be a stress protein or an acute phase protein induced upon cell insult. This is of interest since pancreatic acute phase response, characterized by sudden changes in protein expression is a clear indicator of injury or infections in the pancreatic gland. Since PAP is overexpressed by pancreatic cells in response to cellular stress, it has been evaluated whether serum PAP could be an indicator of different pancreatic diseases. In an initial retrospective study[29], it has been suggested that PAP might be a useful serum marker in the following of acute pancreatitis. In particular, a continuous elevation of serum PAP concentrations indicates that pancreatitis is still in progress. Nevertheless, other studies revealed that despite severity of pancreatitis correlated with serum levels of PAP, the sensitivity of PAP did not allow distinguishing severe from mild acute pancreatitis, better than C reactive protein[30]. In the case of pancreatic cancer, PAP was also overexpressed and could be observed in malignant ductular structures in pancreatic carcinomas[22]. Other reports revealed that PAP was strongly expressed in acini adjacent to the invasive adenocarcinoma, suggesting that the main source of PAP release in the pancreatic juice is acini[23]. Since overexpression of PAP significantly correlated with nodal involvement, distant metastasis, and short survival, it has been suggested that its expression in human pancreatic ductal adenocarcinoma could be an indicator of tumor aggressiveness[22].
PHYSIOLOGICAL FUNCTIONS OF PAP
The fact that PAP is secreted by pancreatic acinar cells into the pancreatic juice initially suggested a role for this protein in pancreatic juice homeostasis. Several data are in line with this. Pancreatic juice is supersaturated in CaCO3 and, in the absence of physiological inhibitors, this salt will precipitate in crystal formation. Interestingly, lithostathine, another member of the PAP protein family, inhibits CaCO3 crystal growth in vitro[31]. On the other hand, it has been shown that PAP can bind and aggregate several bacterial strains from the intestinal flora including Gram positive and Gram negative, aerobic and anaerobic bacteria, although without inhibiting their growth[3]. This fact suggests that PAP could act as an endogenous anti-bacterial agent that could play a protective role preventing infectious complications in acute pancreatitis. Another suggested function for PAP is related to the fact that PAP conferred significant resistance to apoptosis induced by TNFα[32] or by low doses of H2O2[33], indicating that during acute pancreatitis PAP generation could be part of a mechanism of pancreatic cell protection against apoptosis. This anti-apoptotic effect of PAP was also observed in liver in which PAP protects hepatocytes against TNFα-induced apoptosis[24]. An interesting role for PAP has also been observed in motoneurons. It has been reported that PAP expression is activated in developing and regenerating motoneurons[20]. This effect is related to ciliary neurotrophic factor (CNTF), which is an important survival factor for motoneurons. In cultured motoneurons, CNTF induces PAP expression which acts as an autocrine/paracrine neurotrophic factor in a subpopulation of motoneurons, by stimulating a survival pathway involving PI3 kinase, Akt kinase and NFκB[34]. Despite the obvious interest of these observations, it is difficult to link these effects with the enormous amount of PAP released by pancreas during acute pancreatitis or cellular stress. Consequently, other physiological functions, more closely related with pancreatic diseases, have been investigated.
PAP AS AN ANTI-INFLAMMATORY MEDIATOR
The initial indication that PAP could act as a modulat or of the inflammatory response has been provided by Heller and co-workers[35]. Using an ex-vivo model of lung perfusion, these authors observed that PAP reduces the severity of some features associated with N-formyl-methionyl-leucyl-phenyl-alanine (FMLP)-induced activation of leukocytes. This includes the generation of thromboxane B2 and the tissue edema formation in the lung. Since PAP generation has been observed in diseases related with inflammatory processes (pancreatitis, Crohn’s disease, etc.), it was speculated that PAP could act as a cell response to the inflammatory stress. This is in line with the fact that induction in the pancreas of an acute-phase response, in which PAP expression was activated, prior to triggering necrotizing pancreatitis, significantly improved the survival of the animals[36]. Studies demonstrated that the administration of anti-PAP antibodies in an experimental model of taurocholate-induced acute pancreatitis in rats was associated with increased inflammation in the pancreas, evidenced by more abundant necrosis, increased pancreatic myeloperoxidase (MPO) levels and more prominent neutrophil infiltration[37]. Similar results were observed in another study using PAP antisense oligodeoxyribonucleotides to block the expression of PAP prior to induction of pancreatitis. With this approach, pancreatitis-induced PAP expression was reduced by 55% and markers of inflammatory cell damage were increased. These include serum amylase activity, pancreas edema, serum C-reactive protein, leukocyte infiltration and fat necrosis. In addition, the expression of IL-1α, IL-1β and IL-4 was increased in peripheral blood mononuclear cells[38]. Pre-treatment with PAP of the in vitro TNFα-stimulated macrophages results in a dose-dependent inhibition of IL-6 and TNFα mRNA expression[37]. The inhibitory effect of PAP has been observed in different cell lines, including rat pancreatic acinar AR42J cells and human HT29 colon derived cells[39]. In these models, PAP prevented NFκB translocation/activation in response to TNFα, indicating that inhibition by PAP occurs, at least in part, upstream of NFκB pathway. It is of clinical interest that the anti-inflammatory effect of PAP could be observed in other organs than pancreas. For example, increased serum levels of PAP expression has been observed in IBD-affected patients and these levels correlated with clinical and endoscopic parameters. In addition, ex vivo experiments showed that intestinal PAP synthesis and secretion was increased in active IBD and correlated with endoscopic and histological severity of inflammatory lesions. Remarkably, PAP reduced the proinflammatory cytokines secretion of the incubation of intestinal mucosa from active Crohn’s disease in a dose dependent manner[39]. Consequently, it could be suggested that the anti-inflammatory role of PAP is not restricted to the pancreas and could be a more general response of the epithelial cells against the inflammatory processes.
ANTI-INFLAMMATORY ACTIVITY OF PAP IS MEDIATED BY STAT3 ACTIVATION
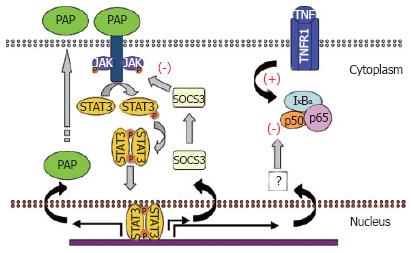
The inhibitory effect of PAP on the pro-inflammatory NFκB pathway seems to be dependent on protein synthesis. This has been demonstrated in AR42J cells by using cycloheximide into the culture medium to prevent de novo protein synthesis[40]. As indicated above, TNFα induces overexpression of the TNFα gene itself and this autocrine loop could be blocked by PAP. However, PAP lost its ability when protein synthesis was inhibited by cycloheximide. This protein synthesis dependence to act as an anti-inflammatory factor shows similarities to IL-10 and IL-6, which depend in part on the synthesis of suppressor of cytokine signalling (SOCS) proteins[41,42]. Both IL-10 and IL-6 receptors are related to the JAK/STAT signal transduction pathways. Therefore, the relationship between PAP and JAK/STAT has also been evaluated. In a recent paper, it has been reported that PAP treatment of the AR42J cells results in a rapid and transient phosphorylation and nuclear translocation of STAT3 protein[40]. In addition, pre-treatment with JAK-specific inhibitors results in the blockage of the inhibitory effects induced by PAP. Finally, the PAP-dependent activation of STAT3 results in a strong activation of its gene target SOCS3 that in turn could be the mediator of the PAP anti-inflammatory effect. Altogether, these results indicate that PAP exerts their effects through the synthesis of a protein induced by the activation of the JAK/STAT signalling pathway. SOCS3 will be the best candidate.
PAP can be strongly induced by IL-6 and IL-10 and IL-10-related cytokines through a STAT3-mediated pathway. Interestingly, expression of PAP itself appears to be induced in pancreatic acinar cells by the presence of PAP in the medium. This is also related to the activation of STAT3 pathway since at least two functional STAT-responsible elements have been reported in the promoter of the PAP gene[43]. This self-induction suggests the existence of a positive feedback mechanism in pancreatic acinar cells via a PAP receptor and a cross-talk with other cytokines (Figure 2).
Figure 2 Anti-inflammatory pathway activated by PAP.
CONCLUDING REMARKS
PAP has been found as a 16 kDa secretory protein specifically induced in pancreas with acute pancreatitis. It has been demonstrated that PAP is activated in some tissues in a constitutive or injury- and inflammation-induced manner. PAP is structurally related to the C-type lectins although not classical lectin-related function has been reported. More recently, it has been found that PAP acts as an anti-inflammatory factor in vitro and in vivo. PAP expression can be induced by several pro- and anti-inflammatory cytokines and by itself through a JAK/STAT3-dependent pathway. PAP is able to activate the expression of the anti-inflammatory factor SOCS3 through the JAK/STAT3-dependent pathway. JAK/STAT3/SOCS3 pathway seems to be a common point between PAP and several cytokines. Therefore, it is reasonable to believe that PAP is a new anti-inflammatory cytokine.
S- Editor Liu Y L- Editor Zhu LH E- Editor Lu W