Published online May 21, 2007. doi: 10.3748/wjg.v13.i19.2752
Revised: March 3, 2007
Accepted: March 21, 2007
Published online: May 21, 2007
AIM: To study the protective effect of grape procyanidins on oxidative injury induced by ethanol and carbon tetrachloride in rat hepatocytes.
METHODS: Normal rat hepatocytes as well as cells damaged by ethanol or carbon tetrachloride were incubated with different doses of grape procyanidins for 24 h. Cell proliferation, apoptosis and TNFα mRNA expression were subsequently determined using MTT assay, cell death ELISA and in situ hybridization.
RESULTS: Proliferative levels of the control cells from ethanol and CCl4 injury groups significantly decreased while apoptosis and TNFα mRNA expression significantly increased compared to the normal control and grape procyanidins co-treatment groups (0.455 ± 0.051 vs 0.318 ± 0.045, P < 0.05). In comparison with the normal control, 50 and 100 mg/L grape procyanidins significantly stimulated cell growth, with a better effect observed with 100 mg/L grape procyanidins.
CONCLUSION: Grape procyanidins inhibit the hepatocyte damage induced by ethanol and carbon tetrachloride, and stimulate normal hepatocyte proliferation.
-
Citation: Zhong JY, Cong HQ, Zhang LH. Inhibitory effects of grape procyanidins on free radical-induced cell damage in rat hepatocytes
in vitro . World J Gastroenterol 2007; 13(19): 2752-2755 - URL: https://www.wjgnet.com/1007-9327/full/v13/i19/2752.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i19.2752
Ethanol and carbon tetrachloride (CCl4) are chemicals that human beings frequently contact during production and habitation. They have hepatic toxicity, and induce hapatocyte injury via various mechanisms including direct effect and free radical formation[1]. Grape procyanidins (GPC) are natural botanic polyphenols extracted from grape seeds, with bioactivities such as antioxidation, free radical elimination and cell proliferation stimulation[2]. In the present study, we used in vitro cultured rat hepatocytes to investigate the inhibitory effects of GPC on ethanol and CCl4 induced hepatocyte injury.
GPC was obtained from Hai Longda Biochemical and Technological Corporation (China), and the content of oligomeric procyanidin was proved to be more than 97% when tested with ultravoilet spectrophoto analysis and hyperbaric liquid chromatography. RPMI 1640 medium, Thiazolyl Blue Tetrazolium Bromide (MTT), typan blue reagent, calf serum oligonucleotide robe, and other reagents for in situ hybridyzation were purchased from Sigma Chemical Corporation. Cell death detection ELISA was obtained from Roche Diagnostics Ltd. Light microscope and phytomicrographic camera PM-CBK-G were products of Olympus, Japan. Computer software Vidas 21 was from Germany. Microplate reader (Bio-Rad model 550) was bought from Sweden.
A Healthy Wistar rat weighing 180 g was exsanguinated to death and rat liver extracted under sterile condition. The liver was triturated using 200 holes metal strainer into hepatocyte suspension, and the cell density was adjusted to 1 × 105 cells/mL. The viability of cells was examined by trypan blue exclusion.
Hepatocytes were divided into three groups: normal control, ethanol injury and CCl4 injury groups. The doses of ethanol and CCl4 were 10 mL/L and 10 mmol/L respectively. Cells received 0, 5, 50 and 100 mg/L of GPC right before ethanol or CCl4 was added.
Fifty μL of cell suspension together with 50 μL of culture medium was added into each well of 96-well plates to reach a cell density of 5 × 104 cells/mL, and then cultured for 12 h at 37°C in humidified atmosphere containing 5% CO2. After incubation, GPC, ethanol and CCl4 were given for 24 h as stated above. Each group had five parallel wells. Cell proliferative activities were subsequently measured using MTT assay. Briefly, after MTT reagent was added to each well and color developed, the absorbance of each sample was measured using a microtitre plate reader, and results were expressed in average optical density (D).
Cell suspension (0.5 mL) and culture medium (0.5 mL) were added into each well of 12-well plates and cultured for 12 h. Cells received various treatment as stated above, and apoptotic levels of each group were indicated by the amount of cytoplasmic histone-associated-DNA fragments and measured using cell death detection ELISA. Basically, peroxidase-conjugated anti-DNA and biotin-labeled anti-histone antibodies were added to the supernatant (containing fragmented DNA) of cell lysate of each sample and reacted with the DNA and histone components of the nucleosomes. The DNA fragmentation was determined quantitatively and photometrically by measuring the amount of peroxidase with ABTs as substrate, and expressed in average optical density (D).
For each well of 6-well plates, 1 mL of cell suspension and 1 mL of culture medium were added to reach a cell density of 5 × 104 cells/mL. A coverslip was placed in advance at the bottom of each well to allow the cells to grow on it. After various treatment as mentioned above, the coverslips (with cells) were taken out and the cells were fixed using paraformaldehyde. After subsequent inactivation of intrinsic enzymes with 3% H2O2, exposure of mRNA nucleotidyl fragments with 3% citric acid diluted pepsin, pre-hybridization, hybridization and coloration, the TNFα mRNA expression level was observed under light microscopy, and quantitatively analysed using Vidas 21 analyzer. For each coverslip, three fields of view were measured and the average amount calculated.
Data were expressed as mean ± standard deviation. All analyses were performed using SPSS 11.0 software and a value of P < 0.05 was considered statistically significant.
Cell proliferative levels of the controls from normal cells, ethanol injury and CCl4 injury groups were 0.448 ± 0.031, 0.232 ± 0.034 and 0.245 ± 0.037, respectively, all of which were significantly lower than the 100 mg GPC co-treated groups (P < 0.05). Other results on cell proliferation are shown in Table 1.
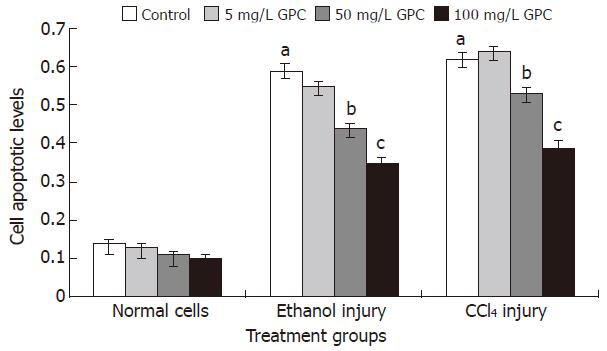
The cell apoptotic levels of the ethanol and CCl4 injury groups were 0.59 ± 0.15 and 0.62 ± 0.13, respectively. With 100 mg/L GPC co-treatment, the cell apoptotic levels of the above two treatment groups significantly dropped to 0.35 ± 0.13 and 0.39 ± 0.14 (P < 0.05). Figure 1 shows the apoptotic levels of each group.
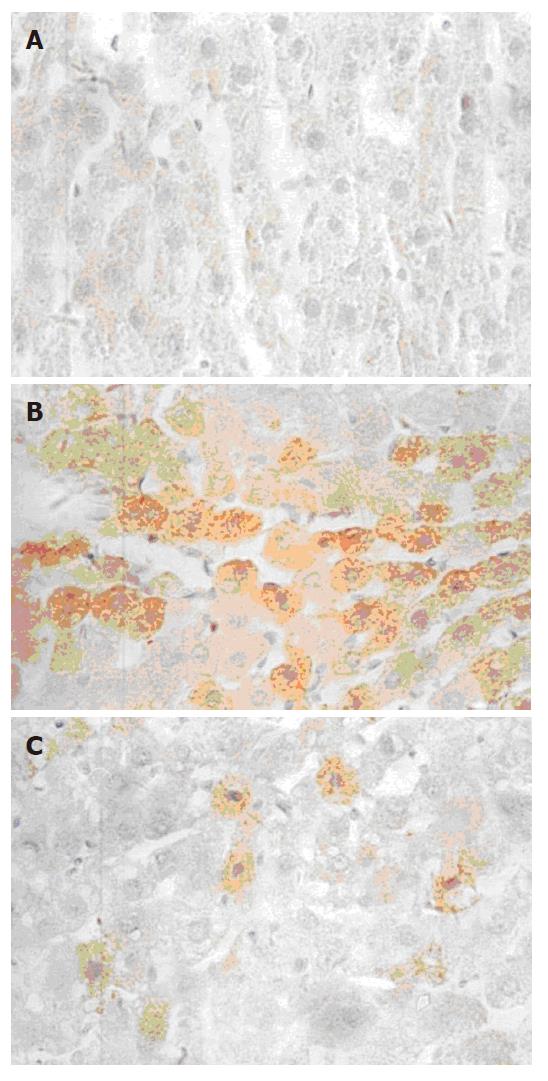
TNFα mRNA was expressed as brown granules in cytoplasm when observed under microscope (× 40). As shown in Figure 2, a large number of cells were observed with brown granules inside and the color was deep in ethanol and CCl4 injury groups, indicating a strong expression of TNFα mRNA. Very few cells from the normal control presented weak TNFα mRNA expression. With 50 and 100 mg/L GPC co-treatment, the TNFα mRNA expression was stronger than the normal control, but weaker than the ethanol and CCl4 injury groups. The TNFα mRNA expression levels (quantitatively analysed by Vidas 21 analyzer) in each group are shown in Table 2.
Liver is an important metabolic organ in human, which has various physiological functions such as biosynthesis, detoxification and excretion. Ethanol and CCl4 are metabolised by liver, from which the free radicals OH- and CCl-3 are formed. These free radicals can cause hepatocyte damage, which leads to abnormal biochemical reactions and molecular structures[1,3].
Cell proliferation is a physiological characteristic of normal cells, and cell apoptosis a physiological cell death which balances the internal environment. Both the cellular proliferative level and apoptotic level are sensitive indexes for evaluating cell activity. When damaged by physical, chemical or biological factors, cells experience decreased proliferation and elevated apoptosis[3,4]. TNFα is a multifunctional cell factor, which plays a role in immunoreactions and internal environment stabilization. It is rarely expressed in normal healthy cells, while when pathological phenomena such as tumor, inflammation and alcoholic hepatitis, occur, the expression of TNFα can be dramatically elevated[5,6].
As shown in the current study, proliferative levels of the control cells from ethanol and CCl4 injury groups significantly decreased, while apoptosis and TNFα mRNA expression significantly increased compared to the normal control cells, indicating that ethanol and CCl4 caused damage to normal healthy hepatocytes. In terms of the GPC effects on ethanol and CCl4-damaged cells, although cells treated with GPC at different doses showed similar changes in proliferation, apoptosis and TNFα mRNA expression to the ethanol and CCl4-treated cells, the injuries were less in extent. The injury levels of the three GPC-treated groups were in order of 5 mg/L > 50 mg/L > 100 mg/L, namely, the injury levels decreased whilst the GPC doses increased. This indicated that GPC efficiently inhibited ethanol and CCl4 induced proliferation inhibition, cell apoptosis and abnormal expression of TNFα mRNA, and it had significant protective effects against hepatocyte injuries. This data is in agreement with the reports on GPC inhibiting free radical damage and gene mutation[7,8], and with our previous studies on GPC inhibiting oxidative stress-induced abnormal expression of endothelial adhesion molecules as well[9].
Furthermore, in terms of the effect of GPC on normal cell proliferation, cells treated with different doses of GPC had higher proliferative activities than the normal control, and an obvious dose-dependent response was obtained: the higher dose of GPC the cells received, the better cell proliferative activity observed. This indicated that GPC promoted cell proliferation in normal hepatocytes and our data is similar to the previous reports on GPC stimulating hair cell growth[10,11]. The stimulating effect of GPC in normal cell proliferation might be associated with its inhibitory effect on endogenous free radicals-generated cell injury, and the precise mechanism needs further investigations.
S- Editor Liu Y L- Editor Ma JY E- Editor Chen GJ
| 1. | Hemmings SJ, Pulga VB, Tran ST, Uwiera RR. Differential inhibitory effects of carbon tetrachloride on the hepatic plasma membrane, mitochondrial and endoplasmic reticular calcium transport systems: implications to hepatotoxicity. Cell Biochem Funct. 2002;20:47-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Bagchi D, Garg A, Krohn RL, Bagchi M, Tran MX, Stohs SJ. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res Commun Mol Pathol Pharmacol. 1997;95:179-189. [PubMed] |
| 3. | Cardin R, D'Errico A, Fiorentino M, Cecchetto A, Naccarato R, Farinati F. Hepatocyte proliferation and apoptosis in relation to oxidative damage in alcohol-related liver disease. Alcohol Alcohol. 2002;37:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Kurose I, Higuchi H, Miura S, Saito H, Watanabe N, Hokari R, Hirokawa M, Takaishi M, Zeki S, Nakamura T. Oxidative stress-mediated apoptosis of hepatocytes exposed to acute ethanol intoxication. Hepatology. 1997;25:368-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 147] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Tong AL, Zeng ZP, Yang D, Li HZ, Li M, Chen S, Sun ML. Expression of transforming growth factor alpha, tumor necrosis factor alpha, and vascular endothelial growth factor of human pheochromocytoma tissues. Zhongguo YiXue KeXueYuan XueBao. 2004;26:426-431. [PubMed] |
| 6. | Kamimura S, Tsukamoto H. Cytokine gene expression by Kupffer cells in experimental alcoholic liver disease. Hepatology. 1995;22:1304-1309. [PubMed] |
| 7. | Li L, Zhong J. Effect of grape procyanidins on the apoptosis and mitochondrial transmembrane potential of thymus cells. WeiSheng YanJiu. 2004;33:191-194. [PubMed] |
| 8. | Castillo J, Benavente-García O, Lorente J, Alcaraz M, Redondo A, Ortuño A, Del Rio JA. Antioxidant activity and radioprotective effects against chromosomal damage induced in vivo by X-rays of flavan-3-ols (Procyanidins) from grape seeds (Vitis vinifera): comparative study versus other phenolic and organic compounds. J Agric Food Chem. 2000;48:1738-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Na N, Zhong JY. Study on the effect of grape procyanidins on cell apoptosis. Weisheng Dulixue. 2002;23:112-114. |
| 10. | Takahashi T, Kamiya T, Hasegawa A, Yokoo Y. Procyanidin oligomers selectively and intensively promote proliferation of mouse hair epithelial cells in vitro and activate hair follicle growth in vivo. J Invest Dermatol. 1999;112:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Takahashi T, Kamimura A, Shirai A, Yokoo Y. Several selective protein kinase C inhibitors including procyanidins promote hair growth. Skin Pharmacol Appl Skin Physiol. 2000;13:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |